Taste of sugar at the membrane: thermodynamics and kinetics of the interaction of a disaccharide with lipid bilayers
- PMID: 23442913
- PMCID: PMC3566452
- DOI: 10.1016/j.bpj.2012.12.011
Taste of sugar at the membrane: thermodynamics and kinetics of the interaction of a disaccharide with lipid bilayers
Abstract
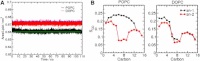
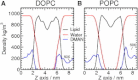
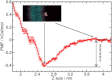
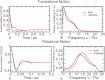
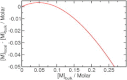
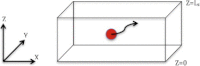
Sugar recognition at the membrane is critical in various physiological processes. Many aspects of sugar-membrane interaction are still unknown. We take an integrated approach by combining conventional molecular-dynamics simulations with enhanced sampling methods and analytical models to understand the thermodynamics and kinetics of a di-mannose molecule in a phospholipid bilayer system. We observe that di-mannose has a slight preference to localize at the water-phospholipid interface. Using umbrella sampling, we show the free energy bias for this preferred location to be just -0.42 kcal/mol, which explains the coexistence of attraction and exclusion mechanisms of sugar-membrane interaction. Accurate estimation of absolute entropy change of water molecules with a two-phase model indicates that the small energy bias is the result of a favorable entropy change of water molecules. Then, we incorporate results from molecular-dynamics simulation in two different ways to an analytical diffusion-reaction model to obtain association and dissociation constants for di-mannose interaction with membrane. Finally, we verify our approach by predicting concentration dependence of di-mannose recognition at the membrane that is consistent with experiment. In conclusion, we provide a combined approach for the thermodynamics and kinetics of a weak ligand-binding system, which has broad implications across many different fields.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Effect of acetone accumulation on structure and dynamics of lipid membranes studied by molecular dynamics simulations.Comput Biol Chem. 2013 Oct;46:23-31. doi: 10.1016/j.compbiolchem.2013.04.005. Epub 2013 May 7. Comput Biol Chem. 2013. PMID: 23764528
-
Free energetics and the role of water in the permeation of methyl guanidinium across the bilayer-water interface: insights from molecular dynamics simulations using charge equilibration potentials.J Phys Chem B. 2013 Apr 4;117(13):3578-92. doi: 10.1021/jp400389z. Epub 2013 Mar 26. J Phys Chem B. 2013. PMID: 23409975
-
Molecular simulation study of phospholipid bilayers and insights of the interactions with disaccharides.Biophys J. 2003 Nov;85(5):2830-44. doi: 10.1016/s0006-3495(03)74706-7. Biophys J. 2003. PMID: 14581188 Free PMC article.
-
Interaction of neurotransmitters with a phospholipid bilayer: a molecular dynamics study.Chem Phys Lipids. 2014 Dec;184:7-17. doi: 10.1016/j.chemphyslip.2014.08.003. Epub 2014 Aug 23. Chem Phys Lipids. 2014. PMID: 25159594
-
Water as a Link between Membrane and Colloidal Theories for Cells.Molecules. 2022 Aug 5;27(15):4994. doi: 10.3390/molecules27154994. Molecules. 2022. PMID: 35956945 Free PMC article. Review.
Cited by
-
Localization of trehalose in partially hydrated DOPC bilayers: insights into cryoprotective mechanisms.J R Soc Interface. 2014 Mar 19;11(95):20140069. doi: 10.1098/rsif.2014.0069. Print 2014 Jun 6. J R Soc Interface. 2014. PMID: 24647907 Free PMC article.
-
The Interaction of Anthracycline Based Quinone-Chelators with Model Lipid Membranes: 1H NMR and MD Study.Membranes (Basel). 2023 Jan 3;13(1):61. doi: 10.3390/membranes13010061. Membranes (Basel). 2023. PMID: 36676868 Free PMC article.
-
A computational study of Anthracyclines interacting with lipid bilayers: Correlation of membrane insertion rates, orientation effects and localisation with cytotoxicity.Sci Rep. 2019 Feb 15;9(1):2155. doi: 10.1038/s41598-019-39411-y. Sci Rep. 2019. PMID: 30770843 Free PMC article.
-
Entropy-Mediated Nanoparticle Cellular Uptake.Small Sci. 2023 Nov 27;4(1):2300078. doi: 10.1002/smsc.202300078. eCollection 2024 Jan. Small Sci. 2023. PMID: 40212624 Free PMC article.
References
-
- Chatterjee D. The mycobacterial cell wall: structure, biosynthesis and sites of drug action. Curr. Opin. Chem. Biol. 1997;1:579–588. - PubMed
-
- Brennan P.J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb.) 2003;83:91–97. - PubMed
-
- Chatterjee D., Hunter S.W., Brennan P.J. Lipoarabinomannan. Multiglycosylated form of the mycobacterial mannosylphosphatidylinositols. J. Biol. Chem. 1992;267:6228–6233. - PubMed
-
- Chatterjee D., Khoo K.H. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8:113–120. - PubMed
-
- Chatterjee D., Lowell K., Brennan P.J. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J. Biol. Chem. 1992;267:6234–6239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

