Membrane interactions and pore formation by the antimicrobial peptide protegrin
- PMID: 23442914
- PMCID: PMC3566446
- DOI: 10.1016/j.bpj.2012.12.038
Membrane interactions and pore formation by the antimicrobial peptide protegrin
Abstract
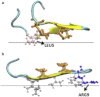
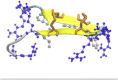
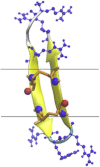
Protegrin is an antimicrobial peptide with a β-hairpin structure stabilized by a pair of disulfide bonds. It has been extensively studied by solid-state NMR and computational methods. Here we use implicit membrane models to examine the binding of monomers on the surface and in the interior of the membrane, the energetics of dimerization, the binding to membrane pores, and the stability of different membrane barrel structures in pores. Our results challenge a number of conclusions based on previous experimental and theoretical work. The burial of monomers into the membrane interior is found to be unfavorable for any membrane thickness. Because of its imperfect amphipathicity, protegrin binds weakly, at most, on the surface of zwitterionic membranes. However, it binds more favorably onto toroidal pores. Anionic charge on the membrane facilitates the binding due to electrostatic interactions. Solid-state NMR results have suggested a parallel NCCN association of monomers in dimers and association of dimers to form octameric or decameric β-barrels. We find that this structure is not energetically plausible for binding to bilayers, because in this configuration the hydrophobic sides of two monomers point in opposite directions. In contrast, the antiparallel NCCN and especially the parallel NCNC octamers are stable and exhibit a favorable binding energy to the pore. The results of 100-ns simulations in explicit bilayers corroborate the higher stability of the parallel NCNC barrel compared with the parallel NCCN barrel. The ability to form pores in zwitterionic membranes provides a rationalization for the peptide's cytotoxicity. The discrepancies between our results and experiment are discussed, and new experiments are proposed to resolve them and to test the validity of the models.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Kokryakov V.N., Harwig S.S.L., Lehrer R.I. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–236. - PubMed
-
- Fahrner R.L., Dieckmann T., Feigon J. Solution structure of protegrin-1, a broad-spectrum antimicrobial peptide from porcine leukocytes. Chem. Biol. 1996;3:543–550. - PubMed
-
- Tam J.P., Wu C.W., Yang J.L. Membranolytic selectivity of cystine-stabilized cyclic protegrins. Eur. J. Biochem. 2000;267:3289–3300. - PubMed
-
- Mangoni M.E., Aumelas A., Chavanieu A. Change in membrane permeability induced by protegrin 1: implication of disulphide bridges for pore formation. FEBS Lett. 1996;383:93–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

