Myosin-II-mediated directional migration of Dictyostelium cells in response to cyclic stretching of substratum
- PMID: 23442953
- PMCID: PMC3576524
- DOI: 10.1016/j.bpj.2013.01.005
Myosin-II-mediated directional migration of Dictyostelium cells in response to cyclic stretching of substratum
Abstract
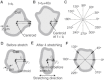
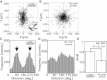
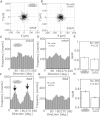
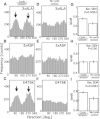
Living cells are constantly subjected to various mechanical stimulations, such as shear flow, osmotic pressure, and hardness of substratum. They must sense the mechanical aspects of their environment and respond appropriately for proper cell function. Cells adhering to substrata must receive and respond to mechanical stimuli from the substrata to decide their shape and/or migrating direction. In response to cyclic stretching of the elastic substratum, intracellular stress fibers in fibroblasts and endothelial, osteosarcoma, and smooth muscle cells are rearranged perpendicular to the stretching direction, and the shape of those cells becomes extended in this new direction. In the case of migrating Dictyostelium cells, cyclic stretching regulates the direction of migration, and not the shape, of the cell. The cells migrate in a direction perpendicular to that of the stretching. However, the molecular mechanisms that induce the directional migration remain unknown. Here, using a microstretching device, we recorded green fluorescent protein (GFP)-myosin-II dynamics in Dictyostelium cells on an elastic substratum under cyclic stretching. Repeated stretching induced myosin II localization equally on both stretching sides in the cells. Although myosin-II-null cells migrated randomly, myosin-II-null cells expressing a variant of myosin II that cannot hydrolyze ATP migrated perpendicular to the stretching. These results indicate that Dictyostelium cells accumulate myosin II at the portion of the cell where a large strain is received and migrate in a direction other than that of the portion where myosin II accumulated. This polarity generation for migration does not require the contraction of actomyosin.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Giannone G., Sheetz M.P. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. - PubMed
-
- Vogel V., Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. - PubMed
-
- Naruse K., Yamada T., Sokabe M. Pp125FAK is required for stretch dependent morphological response of endothelial cells. Oncogene. 1998;17:455–463. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

