Live-cell superresolution imaging by pulsed STED two-photon excitation microscopy
- PMID: 23442955
- PMCID: PMC3576532
- DOI: 10.1016/j.bpj.2012.12.053
Live-cell superresolution imaging by pulsed STED two-photon excitation microscopy
Abstract
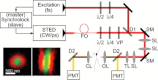
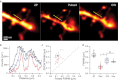
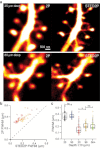
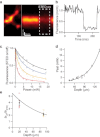
Two-photon laser scanning microscopy (2PLSM) allows fluorescence imaging in thick biological samples where absorption and scattering typically degrade resolution and signal collection of one-photon imaging approaches. The spatial resolution of conventional 2PLSM is limited by diffraction, and the near-infrared wavelengths used for excitation in 2PLSM preclude the accurate imaging of many small subcellular compartments of neurons. Stimulated emission depletion (STED) microscopy is a superresolution imaging modality that overcomes the resolution limit imposed by diffraction and allows fluorescence imaging of nanoscale features. Here, we describe the design and operation of a superresolution two-photon microscope using pulsed excitation and STED lasers. We examine the depth dependence of STED imaging in acute tissue slices and find enhancement of 2P resolution ranging from approximately fivefold at 20 μm to approximately twofold at 90-μm deep. The depth dependence of resolution is found to be consistent with the depth dependence of depletion efficiency, suggesting resolution is limited by STED laser propagation through turbid tissue. Finally, we achieve live imaging of dendritic spines with 60-nm resolution and demonstrate that our technique allows accurate quantification of neuronal morphology up to 30-μm deep in living brain tissue.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Superresolving dendritic spines.Biophys J. 2013 Feb 19;104(4):741-3. doi: 10.1016/j.bpj.2013.01.011. Biophys J. 2013. PMID: 23442950 Free PMC article. No abstract available.
References
-
- Denk W., Strickler J.H., Webb W.W. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. - PubMed
-
- Svoboda K., Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–839. - PubMed
-
- Hell S.W., Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994;19:780–782. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

