Characterization of a ligand binding site in the human transient receptor potential ankyrin 1 pore
- PMID: 23442958
- PMCID: PMC3576529
- DOI: 10.1016/j.bpj.2013.01.008
Characterization of a ligand binding site in the human transient receptor potential ankyrin 1 pore
Abstract
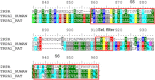
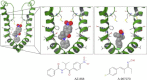
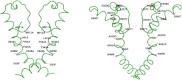
The pharmacology and regulation of Transient Receptor Potential Ankyrin 1 (TRPA1) ion channel activity is intricate due to the physiological function as an integrator of multiple chemical, mechanical, and temperature stimuli as well as differences in species pharmacology. In this study, we describe and compare the current inhibition efficacy of human TRPA1 on three different TRPA1 antagonists. We used a homology model of TRPA1 based on Kv1.2 to select pore vestibule residues available for interaction with ligands entering the vestibule. Site-directed mutation constructs were expressed in Xenopus oocytes and their functionality and pharmacology assessed to support and improve our homology model. Based on the functional pharmacology results we propose an antagonist-binding site in the vestibule of the TRPA1 ion channel. We use the results to describe the proposed intravestibular ligand-binding site in TRPA1 in detail. Based on the single site substitutions, we designed a human TRPA1 receptor by substituting several residues in the vestibule and adjacent regions from the rat receptor to address and explain observed species pharmacology differences. In parallel, the lack of effect on HC-030031 inhibition by the vestibule substitutions suggests that this molecule interacts with TRPA1 via a binding site not situated in the vestibule.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


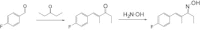
References
-
- Bandell M., Story G.M., Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. - PubMed
-
- Story G.M., Peier A.M., Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

