Intramolecular proton transfer in channelrhodopsins
- PMID: 23442959
- PMCID: PMC3576534
- DOI: 10.1016/j.bpj.2013.01.002
Intramolecular proton transfer in channelrhodopsins
Abstract
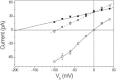
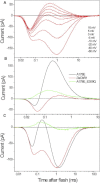
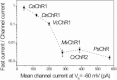
Channelrhodopsins serve as photoreceptors that control the motility behavior of green flagellate algae and act as light-gated ion channels when heterologously expressed in animal cells. Here, we report direct measurements of proton transfer from the retinylidene Schiff base in several channelrhodopsin variants expressed in HEK293 cells. A fast outward-directed current precedes the passive channel current that has the opposite direction at physiological holding potentials. This rapid charge movement occurs on the timescale of the M intermediate formation in microbial rhodopsins, including that for channelrhodopsin from Chlamydomonas augustae and its mutants, reported in this study. Mutant analysis showed that the glutamate residue corresponding to Asp(85) in bacteriorhodopsin acts as the primary acceptor of the Schiff-base proton in low-efficiency channelrhodopsins. Another photoactive-site residue corresponding to Asp(212) in bacteriorhodopsin serves as an alternative proton acceptor and plays a more important role in channel opening than the primary acceptor. In more efficient channelrhodopsins from Chlamydomonas reinhardtii, Mesostigma viride, and Platymonas (Tetraselmis) subcordiformis, the fast current was apparently absent. The inverse correlation of the outward proton transfer and channel activity is consistent with channel function evolving in channelrhodopsins at the expense of their capacity for active proton transport.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures







Comment in
-
A thin line between channels and pumps.Biophys J. 2013 Feb 19;104(4):739-40. doi: 10.1016/j.bpj.2012.12.050. Biophys J. 2013. PMID: 23442949 Free PMC article. No abstract available.
Similar articles
-
Bacteriorhodopsin-like channelrhodopsins: Alternative mechanism for control of cation conductance.Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):E9512-E9519. doi: 10.1073/pnas.1710702114. Epub 2017 Oct 25. Proc Natl Acad Sci U S A. 2017. PMID: 29078348 Free PMC article.
-
Retinal chromophore structure and Schiff base interactions in red-shifted channelrhodopsin-1 from Chlamydomonas augustae.Biochemistry. 2014 Jun 24;53(24):3961-70. doi: 10.1021/bi500445c. Epub 2014 Jun 16. Biochemistry. 2014. PMID: 24869998 Free PMC article.
-
Channelrhodopsin unchained: structure and mechanism of a light-gated cation channel.Biochim Biophys Acta. 2014 May;1837(5):626-42. doi: 10.1016/j.bbabio.2013.10.014. Epub 2013 Nov 7. Biochim Biophys Acta. 2014. PMID: 24212055 Review.
-
Role of a helix B lysine residue in the photoactive site in channelrhodopsins.Biophys J. 2014 Apr 15;106(8):1607-17. doi: 10.1016/j.bpj.2014.03.002. Biophys J. 2014. PMID: 24739160 Free PMC article.
-
Mechanism divergence in microbial rhodopsins.Biochim Biophys Acta. 2014 May;1837(5):546-52. doi: 10.1016/j.bbabio.2013.06.006. Epub 2013 Jul 3. Biochim Biophys Acta. 2014. PMID: 23831552 Free PMC article. Review.
Cited by
-
Kinetic and vibrational isotope effects of proton transfer reactions in channelrhodopsin-2.Biophys J. 2015 Jul 21;109(2):287-97. doi: 10.1016/j.bpj.2015.06.023. Biophys J. 2015. PMID: 26200864 Free PMC article.
-
Sodium-Selective Channelrhodopsins.Cells. 2024 Nov 8;13(22):1852. doi: 10.3390/cells13221852. Cells. 2024. PMID: 39594600 Free PMC article. Review.
-
Mechanism by which water and protein electrostatic interactions control proton transfer at the active site of channelrhodopsin.PLoS One. 2018 Aug 7;13(8):e0201298. doi: 10.1371/journal.pone.0201298. eCollection 2018. PLoS One. 2018. PMID: 30086158 Free PMC article.
-
Femtosecond infrared spectroscopy of channelrhodopsin-1 chromophore isomerization.Struct Dyn. 2016 Apr 29;3(4):043208. doi: 10.1063/1.4948338. eCollection 2016 Jul. Struct Dyn. 2016. PMID: 27191011 Free PMC article.
-
Potassium-selective channelrhodopsins.Biophys Physicobiol. 2023 Feb 4;20(Supplemental):e201011. doi: 10.2142/biophysico.bppb-v20.s011. eCollection 2023 Mar 21. Biophys Physicobiol. 2023. PMID: 38362336 Free PMC article.
References
-
- Spudich J.L., Jung K.-H. Handbook of Photosensory Receptors. Wiley-VCH; Weinheim, Germany: 2005. Microbial rhodopsins: phylogenetic and functional diversity; pp. 1–23.
-
- Litvin F.F., Sineshchekov O.A., Sineshchekov V.A. Photoreceptor electric potential in the phototaxis of the alga Haematococcus pluvialis. Nature. 1978;271:476–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

