Anthocyanins and their variation in red wines. II. Anthocyanin derived pigments and their color evolution
- PMID: 23442981
- PMCID: PMC6269080
- DOI: 10.3390/molecules17021483
Anthocyanins and their variation in red wines. II. Anthocyanin derived pigments and their color evolution
Abstract
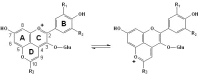
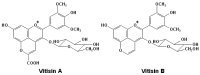
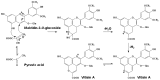
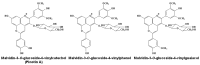
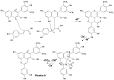
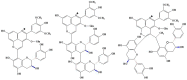
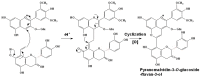
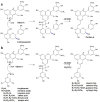
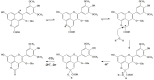
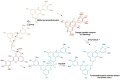
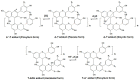
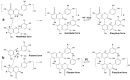
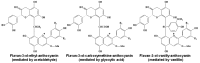
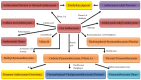
Originating in the grapes, anthocyanins and their derivatives are the crucial pigments responsible for the red wine color. During wine maturation and aging, the concentration of monomeric anthocyanins declines constantly, while numerous more complex and stable anthocyanin derived pigments are formed, mainly including pyranoanthocyanins, polymeric anthocyanins produced from condensation between anthocyanin and/or flavan-3-ols directly or mediated by aldehydes. Correspondingly, their structural modifications result in a characteristic variation of color, from purple-red color in young red wines to brick-red hue of the aged. Because of the extreme complexity of chemical compounds involved, many investigations have been made using model solutions of know composition rather than wine. Thus, there is a large amount of research still required to obtain an overall perspective of the anthocyanin composition and its change with time in red wines. Future findings may well greatly revise our current interpretation of the color in red wines. This paper summarizes the most recent advances in the studies of the anthocyanins derived pigments in red wines, as well as their color evolution.
Figures




Similar articles
-
Identification, content and distribution of anthocyanins and low molecular weight anthocyanin-derived pigments in Spanish commercial red wines.Food Chem. 2014 Sep 1;158:449-58. doi: 10.1016/j.foodchem.2014.02.154. Epub 2014 Mar 10. Food Chem. 2014. PMID: 24731369
-
Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression.Molecules. 2012 Feb 7;17(2):1571-601. doi: 10.3390/molecules17021571. Molecules. 2012. PMID: 22314380 Free PMC article. Review.
-
Reaction Kinetics of Monomeric Anthocyanin Conversion to Polymeric Pigments and Their Significance to Color in Interspecific Hybrid Wines.J Agric Food Chem. 2017 Aug 9;65(31):6379-6386. doi: 10.1021/acs.jafc.6b05331. Epub 2017 Feb 15. J Agric Food Chem. 2017. PMID: 28171723
-
Pectin forms polymeric pigments by complexing anthocyanins during red winemaking and ageing.Food Res Int. 2024 Jul;188:114442. doi: 10.1016/j.foodres.2024.114442. Epub 2024 May 5. Food Res Int. 2024. PMID: 38823830
-
Formation of pyranoanthocyanins in red wines: a new and diverse class of anthocyanin derivatives.Anal Bioanal Chem. 2011 Sep;401(5):1463-73. doi: 10.1007/s00216-010-4479-9. Epub 2010 Dec 22. Anal Bioanal Chem. 2011. PMID: 21181135 Review.
Cited by
-
Toasted Vine Shoots as an Alternative Enological Tool. Impact on the Sensory Profile of Tempranillo Wines during Bottle Aging.J Agric Food Chem. 2024 Jan 31;72(4):1914-1927. doi: 10.1021/acs.jafc.2c08982. Epub 2023 Mar 24. J Agric Food Chem. 2024. PMID: 36960639 Free PMC article.
-
Miniaturized electrocoagulation approach for removal of polymeric pigments and selective analysis of non- and mono-hydroxylated phenolic acids in wine with HPLC-UV.RSC Adv. 2021 Feb 2;11(11):5885-5893. doi: 10.1039/d0ra09089a. eCollection 2021 Feb 2. RSC Adv. 2021. PMID: 35814731 Free PMC article.
-
The key role of vineyard parcel in shaping flavonoid profiles and color characteristics of Cabernet Sauvignon wines combined with the influence of harvest ripeness, vintage and bottle aging.Food Chem X. 2023 Jun 25;19:100772. doi: 10.1016/j.fochx.2023.100772. eCollection 2023 Oct 30. Food Chem X. 2023. PMID: 37780257 Free PMC article.
-
Surface-displayed phenolic acid decarboxylase for increased vinylphenolic pyranoanthocyanins in blueberry wine.Curr Res Food Sci. 2024 Apr 7;8:100730. doi: 10.1016/j.crfs.2024.100730. eCollection 2024. Curr Res Food Sci. 2024. PMID: 38623272 Free PMC article.
-
Untargeted Metabolomics Analysis Based on LC-QTOF-MS to Investigate the Phenolic Composition of Red and White Wines Elaborated from Sonicated Grapes.Foods. 2024 Jun 4;13(11):1761. doi: 10.3390/foods13111761. Foods. 2024. PMID: 38890989 Free PMC article.
References
-
- Wrolstad R.E., Durst R.W., Lee J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Tech. 2005;16:423–428. doi: 10.1016/j.tifs.2005.03.019. - DOI
-
- Degenhardt A., Hofmann S., Knapp H., Winterhalter P. Preparative isolation of anthocyanins by high-speed countercurrent chromatography and application of the color activity concept to red wine. J. Agric. Food Chem. 2000;48:5812–5818. - PubMed
-
- Robinson W.B., Weirs L.D., Bertino J.J., Mattick L.R. The relation of anthocyanin composition to color stability of New York State wines. Am. J. Enol. Vitic. 1966;17:178–184.
-
- Busse-Valverde N., Gómez-Plaza E., López-Roc J.M., Gil-Muňoz R., Bautista-Ortín A.B. The extraction of anthocyanins and proanthocyanidins from grapes to wine during fermentative maceration is affected by the enological technique. J. Agric. Food Chem. 2011;59:5450–5455. doi: 10.1021/jf2002188. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

