Photothermal nanodrugs: potential of TNF-gold nanospheres for cancer theranostics
- PMID: 23443065
- PMCID: PMC3582999
- DOI: 10.1038/srep01293
Photothermal nanodrugs: potential of TNF-gold nanospheres for cancer theranostics
Abstract
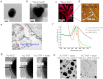
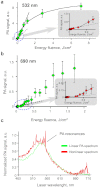
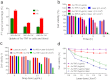
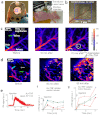
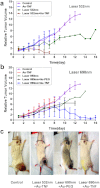
Nanotechnology has been extensively explored for drug delivery. Here, we introduce the concept of a nanodrug based on synergy of photothermally-activated physical and biological effects in nanoparticle-drug conjugates. To prove this concept, we utilized tumor necrosis factor-alpha coated gold nanospheres (Au-TNF) heated by laser pulses. To enhance photothermal efficiency in near-infrared window of tissue transparency we explored slightly ellipsoidal nanoparticles, its clustering, and laser-induced nonlinear dynamic phenomena leading to amplification and spectral sharpening of photothermal and photoacoustic resonances red-shifted relatively to linear plasmonic resonances. Using a murine carcinoma model, we demonstrated higher therapy efficacy of Au-TNF conjugates compared to laser and Au-TNF alone or laser with TNF-free gold nanospheres. The photothermal activation of low toxicity Au-TNF conjugates, which are in phase II trials in humans, with a laser approved for medical applications opens new avenues in the development of clinically relevant nanodrugs with synergistic antitumor theranostic action.
Figures

References
-
- Agarwal A., Mackey M. A., El-Sayed M. A. & Bellamkonda R. V. Remote triggered release of doxorubicin in tumors by synergistic application of thermosensitive liposomes and gold nanorods. ACS Nano 5, 4919–4926 (2011). - PubMed
-
- Farokhzad O. C. & Langer R. Impact of nanotechnology on drug delivery. ACS Nano 3, 16–20 (2009). - PubMed
-
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 5, 161–171 (2005). - PubMed
-
- Koo O. M., Rubinstein I. & Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine: Nanotechnol, Biology and Medicine 1, 193–212 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

