Insulin signalling mechanisms for triacylglycerol storage
- PMID: 23443243
- PMCID: PMC3652374
- DOI: 10.1007/s00125-013-2869-1
Insulin signalling mechanisms for triacylglycerol storage
Abstract
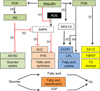
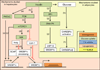
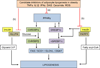
Insulin signalling is uniquely required for storing energy as fat in humans. While de novo synthesis of fatty acids and triacylglycerol occurs mostly in liver, adipose tissue is the primary site for triacylglycerol storage. Insulin signalling mechanisms in adipose tissue that stimulate hydrolysis of circulating triacylglycerol, uptake of the released fatty acids and their conversion to triacylglycerol are poorly understood. New findings include (1) activation of DNA-dependent protein kinase to stimulate upstream stimulatory factor (USF)1/USF2 heterodimers, enhancing the lipogenic transcription factor sterol regulatory element binding protein 1c (SREBP1c); (2) stimulation of fatty acid synthase through AMP kinase modulation; (3) mobilisation of lipid droplet proteins to promote retention of triacylglycerol; and (4) upregulation of a novel carbohydrate response element binding protein β isoform that potently stimulates transcription of lipogenic enzymes. Additionally, insulin signalling through mammalian target of rapamycin to activate transcription and processing of SREBP1c described in liver may apply to adipose tissue. Paradoxically, insulin resistance in obesity and type 2 diabetes is associated with increased triacylglycerol synthesis in liver, while it is decreased in adipose tissue. This and other mysteries about insulin signalling and insulin resistance in adipose tissue make this topic especially fertile for future research.
Figures

References
-
- Tattersall RB. A force of magical activity: the introduction of insulin treatment in Britain 1922–1926. Diabet Med. 1995;12:739–755. - PubMed
-
- Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87:81–86. - PubMed
-
- Frayn KN. Regulation of fatty acid delivery in vivo. Adv Exp Med Biol. 1998;441:171–179. - PubMed
-
- Frayn KN. Adipose tissue and the insulin resistance syndrome. Proc Nutr Soc. 2001;60:375–380. - PubMed
-
- Boden G. Free fatty acids-the link between obesity and insulin resistance. Endocr Pract. 2001;7:44–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

