Metal-catalyzed double migratory cascade reactions of propargylic esters and phosphates
- PMID: 23443274
- PMCID: PMC3678355
- DOI: 10.1039/c3cs35514d
Metal-catalyzed double migratory cascade reactions of propargylic esters and phosphates
Abstract
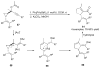
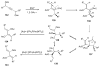
Propargylic esters and phosphates are easily accessible substrates, which exhibit rich and tunable reactivities in the presence of transition metal catalysts. π-Acidic metals, mostly gold and platinum salts, activate these substrates for an initial 1,2- or 1,3-acyloxy and phosphatyloxy migration process to form reactive intermediates. These intermediates are able to undergo further cascade reactions leading to a variety of diverse structures. This tutorial review systematically introduces the double migratory reactions of propargylic esters and phosphates as a novel synthetic method, in which further cascade reaction of the reactive intermediate is accompanied by a second migration of a different group, thus offering a rapid route to a wide range of functionalized products. The serendipitous observations, as well as designed approaches involving the double migratory cascade reactions, will be discussed with emphasis placed on the mechanistic aspects and the synthetic utilities of the obtained products.
Figures










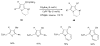






















References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

