Sensory nerve terminal mitochondrial dysfunction activates airway sensory nerves via transient receptor potential (TRP) channels
- PMID: 23444014
- PMCID: PMC3629826
- DOI: 10.1124/mol.112.084319
Sensory nerve terminal mitochondrial dysfunction activates airway sensory nerves via transient receptor potential (TRP) channels
Abstract
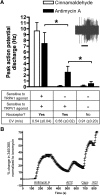
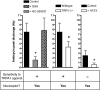
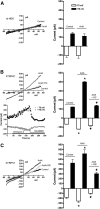
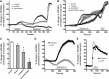
Mitochondrial dysfunction and subsequent oxidative stress has been reported for a variety of cell types in inflammatory diseases. Given the abundance of mitochondria at the peripheral terminals of sensory nerves and the sensitivity of transient receptor potential (TRP) ankyrin 1 (A1) and TRP vanilloid 1 (V1) to reactive oxygen species (ROS) and their downstream products of lipid peroxidation, we investigated the effect of nerve terminal mitochondrial dysfunction on airway sensory nerve excitability. Here we show that mitochondrial dysfunction evoked by acute treatment with antimycin A (mitochondrial complex III Qi site inhibitor) preferentially activated TRPA1-expressing "nociceptor-like" mouse bronchopulmonary C-fibers. Action potential discharge was reduced by the TRPA1 antagonist HC-030031. Inhibition of TRPV1 further reduced C-fiber activation. In mouse dissociated vagal neurons, antimycin A induced Ca(2+) influx that was significantly reduced by pharmacological inhibition or genetic knockout of either TRPA1 or TRPV1. Inhibition of both TRPA1 and TRPV1 was required to abolish antimycin A-induced Ca(2+) influx in vagal neurons. Using an HEK293 cell expression system, antimycin A induced concentration-dependent activation of both hTRPA1 and hTRPV1 but failed to activate nontransfected cells. Myxothiazol (complex III Qo site inhibitor) inhibited antimycin A-induced TRPA1 activation, as did the reducing agent dithiothreitol. Scavenging of both superoxide and hydrogen peroxide inhibited TRPA1 activation following mitochondrial modulation. In conclusion, we present evidence that acute mitochondrial dysfunction activates airway sensory nerves preferentially via TRPA1 through the actions of mitochondrially-derived ROS. This represents a novel mechanism by which inflammation may be transduced into nociceptive electrical signaling.
Figures


Similar articles
-
Antimycin A-induced mitochondrial dysfunction activates vagal sensory neurons via ROS-dependent activation of TRPA1 and ROS-independent activation of TRPV1.Brain Res. 2019 Jul 15;1715:94-105. doi: 10.1016/j.brainres.2019.03.029. Epub 2019 Mar 23. Brain Res. 2019. PMID: 30914247 Free PMC article.
-
Mitochondrial modulation-induced activation of vagal sensory neuronal subsets by antimycin A, but not CCCP or rotenone, correlates with mitochondrial superoxide production.PLoS One. 2018 May 7;13(5):e0197106. doi: 10.1371/journal.pone.0197106. eCollection 2018. PLoS One. 2018. PMID: 29734380 Free PMC article.
-
Sensory nerve terminal mitochondrial dysfunction induces hyperexcitability in airway nociceptors via protein kinase C.Mol Pharmacol. 2014 Jun;85(6):839-48. doi: 10.1124/mol.113.091272. Epub 2014 Mar 18. Mol Pharmacol. 2014. PMID: 24642367 Free PMC article.
-
Interaction between TRPA1 and TRPV1: Synergy on pulmonary sensory nerves.Pulm Pharmacol Ther. 2015 Dec;35:87-93. doi: 10.1016/j.pupt.2015.08.003. Epub 2015 Aug 14. Pulm Pharmacol Ther. 2015. PMID: 26283426 Free PMC article. Review.
-
Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions.Curr Pharm Biotechnol. 2011 Jan 1;12(1):89-94. doi: 10.2174/138920111793937952. Curr Pharm Biotechnol. 2011. PMID: 20932255 Review.
Cited by
-
Diagnosis and treatments for oropharyngeal dysphagia: effects of capsaicin evaluated by newly developed ultrasonographic method.J Smooth Muscle Res. 2020;56(0):46-57. doi: 10.1540/jsmr.56.46. J Smooth Muscle Res. 2020. PMID: 32581185 Free PMC article. Review.
-
Mapping of the Sensory Innervation of the Mouse Lung by Specific Vagal and Dorsal Root Ganglion Neuronal Subsets.eNeuro. 2022 Apr 13;9(2):ENEURO.0026-22.2022. doi: 10.1523/ENEURO.0026-22.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35365503 Free PMC article.
-
A melatonin-based fluorescence method for the measurement of mitochondrial complex III function in intact cells.J Pineal Res. 2013 Nov;55(4):364-70. doi: 10.1111/jpi.12079. Epub 2013 Aug 17. J Pineal Res. 2013. PMID: 23952718 Free PMC article.
-
Human TRPM8 and TRPA1 pain channels, including a gene variant with increased sensitivity to agonists (TRPA1 R797T), exhibit differential regulation by SRC-tyrosine kinase inhibitor.Biosci Rep. 2014 Aug 6;34(4):e00131. doi: 10.1042/BSR20140061. Biosci Rep. 2014. PMID: 24975826 Free PMC article.
-
TRPA1 channels: molecular sentinels of cellular stress and tissue damage.J Physiol. 2016 Aug 1;594(15):4151-69. doi: 10.1113/JP270935. J Physiol. 2016. PMID: 27079970 Free PMC article. Review.
References
-
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

