Probing the metabotropic glutamate receptor 5 (mGlu₅) positive allosteric modulator (PAM) binding pocket: discovery of point mutations that engender a "molecular switch" in PAM pharmacology
- PMID: 23444015
- PMCID: PMC3629835
- DOI: 10.1124/mol.112.083949
Probing the metabotropic glutamate receptor 5 (mGlu₅) positive allosteric modulator (PAM) binding pocket: discovery of point mutations that engender a "molecular switch" in PAM pharmacology
Abstract
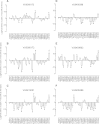
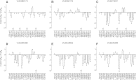
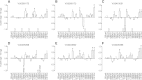
Positive allosteric modulation of metabotropic glutamate receptor subtype 5 (mGlu₅) is a promising novel approach for the treatment of schizophrenia and cognitive disorders. Allosteric binding sites are topographically distinct from the endogenous ligand (orthosteric) binding site, allowing for co-occupation of a single receptor with the endogenous ligand and an allosteric modulator. Negative allosteric modulators (NAMs) inhibit and positive allosteric modulators (PAMs) enhance the affinity and/or efficacy of the orthosteric agonist. The molecular determinants that govern mGlu₅ modulator affinity versus cooperativity are not well understood. Focusing on the modulators based on the acetylene scaffold, we sought to determine the molecular interactions that contribute to PAM versus NAM pharmacology. Generation of a comparative model of the transmembrane-spanning region of mGlu₅ served as a tool to predict and interpret the impact of mutations in this region. Application of an operational model of allosterism allowed for determination of PAM and NAM affinity estimates at receptor constructs that possessed no detectable radioligand binding as well as delineation of effects on affinity versus cooperativity. Novel mutations within the transmembrane domain (TM) regions were identified that had differential effects on acetylene PAMs versus 2-methyl-6-(phenylethynyl)-pyridine, a prototypical NAM. Three conserved amino acids (Y658, T780, and S808) and two nonconserved residues (P654 and A809) were identified as key determinants of PAM activity. Interestingly, we identified two point mutations in TMs 6 and 7 that, when mutated, engender a mode switch in the pharmacology of certain PAMs.
Figures
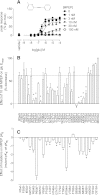
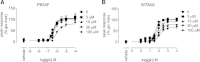
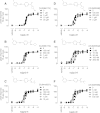



Similar articles
-
Investigating metabotropic glutamate receptor 5 allosteric modulator cooperativity, affinity, and agonism: enriching structure-function studies and structure-activity relationships.Mol Pharmacol. 2012 Nov;82(5):860-75. doi: 10.1124/mol.112.080531. Epub 2012 Aug 3. Mol Pharmacol. 2012. PMID: 22863693 Free PMC article.
-
Probe dependence and biased potentiation of metabotropic glutamate receptor 5 is mediated by differential ligand interactions in the common allosteric binding site.Biochem Pharmacol. 2020 Jul;177:114013. doi: 10.1016/j.bcp.2020.114013. Epub 2020 May 8. Biochem Pharmacol. 2020. PMID: 32389635
-
Structural determinants of allosteric antagonism at metabotropic glutamate receptor 2: mechanistic studies with new potent negative allosteric modulators.Br J Pharmacol. 2011 Sep;164(2b):521-37. doi: 10.1111/j.1476-5381.2011.01409.x. Br J Pharmacol. 2011. PMID: 21470207 Free PMC article.
-
Pharmacological and molecular characterization of the positive allosteric modulators of metabotropic glutamate receptor 2.Neuropharmacology. 2016 Dec;111:253-265. doi: 10.1016/j.neuropharm.2016.08.032. Epub 2016 Aug 30. Neuropharmacology. 2016. PMID: 27590915 Review.
-
Reprint of Pharmacological and molecular characterization of the positive allosteric modulators of metabotropic glutamate receptor 2.Neuropharmacology. 2017 Mar 15;115:115-127. doi: 10.1016/j.neuropharm.2016.08.040. Epub 2017 Feb 16. Neuropharmacology. 2017. PMID: 28216000 Review.
Cited by
-
Biased allosteric agonism and modulation of metabotropic glutamate receptor 5: Implications for optimizing preclinical neuroscience drug discovery.Neuropharmacology. 2017 Mar 15;115:60-72. doi: 10.1016/j.neuropharm.2016.07.001. Epub 2016 Jul 5. Neuropharmacology. 2017. PMID: 27392634 Free PMC article.
-
Molecular Insights into Metabotropic Glutamate Receptor Allosteric Modulation.Mol Pharmacol. 2015 Jul;88(1):188-202. doi: 10.1124/mol.114.097220. Epub 2015 Mar 25. Mol Pharmacol. 2015. PMID: 25808929 Free PMC article.
-
Practical Strategies and Concepts in GPCR Allosteric Modulator Discovery: Recent Advances with Metabotropic Glutamate Receptors.Chem Rev. 2016 Jun 8;116(11):6707-41. doi: 10.1021/acs.chemrev.5b00656. Epub 2016 Feb 16. Chem Rev. 2016. PMID: 26882314 Free PMC article. Review.
-
Stepwise activation of a metabotropic glutamate receptor.Nature. 2024 May;629(8013):951-956. doi: 10.1038/s41586-024-07327-x. Epub 2024 Apr 17. Nature. 2024. PMID: 38632403 Free PMC article.
-
mGluR5: exploration of orthosteric and allosteric ligand binding pockets and their applications to drug discovery.Neurochem Res. 2014 Oct;39(10):1862-75. doi: 10.1007/s11064-014-1248-8. Epub 2014 Feb 4. Neurochem Res. 2014. PMID: 24493625
References
-
- Alexander N, Woetzel N, and Meiler J (2011) Bcl::Cluster: a method for clustering biological molecules coupled with visualization in the Pymol Molecular Graphics System, in Proceedings of the 2011 IEEE 1st International Conference on Computational Advances in Bio and Medical Sciences; 2011 February 3–5; Orlando, FL. pp 13–18, IEEE Computer Society, Los Alamitos. - PMC - PubMed
-
- Ametamey SM, Treyer V, Streffer J, Wyss MT, Schmidt M, Blagoev M, Hintermann S, Auberson Y, Gasparini F, Fischer UC, et al. (2007) Human PET studies of metabotropic glutamate receptor subtype 5 with 11C-ABP688. J Nucl Med 48:247–252 - PubMed
-
- Barak LS, Ménard L, Ferguson SS, Colapietro AM, Caron MG. (1995) The conserved seven-transmembrane sequence NP(X)2,3Y of the G-protein-coupled receptor superfamily regulates multiple properties of the beta 2-adrenergic receptor. Biochemistry 34:15407–15414 - PubMed
-
- Chen Y, Goudet C, Pin JP, Conn PJ. (2008) N-4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol 73:909–918 - PubMed
-
- Chen Y, Nong Y, Goudet C, Hemstapat K, de Paulis T, Pin JP, Conn PJ. (2007) Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol Pharmacol 71:1389–1398 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM099842/GM/NIGMS NIH HHS/United States
- R01 MH062646/MH/NIMH NIH HHS/United States
- 5 U54 MH84659-03/MH/NIMH NIH HHS/United States
- R01-GM099842/GM/NIGMS NIH HHS/United States
- 2R01-NS031373-16A2/NS/NINDS NIH HHS/United States
- R01 NS031373/NS/NINDS NIH HHS/United States
- 1R01-DA023947/DA/NIDA NIH HHS/United States
- 5 U54 MH84659-03S1/MH/NIMH NIH HHS/United States
- R01 MH090192/MH/NIMH NIH HHS/United States
- R01-GM080403/GM/NIGMS NIH HHS/United States
- U19 MH097056/MH/NIMH NIH HHS/United States
- R01-MH090192/MH/NIMH NIH HHS/United States
- U54 MH084659/MH/NIMH NIH HHS/United States
- R01 GM080403/GM/NIGMS NIH HHS/United States
- 2R01-MH062646-13/MH/NIMH NIH HHS/United States
- R01 DA023947/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

