Chemokines in innate and adaptive granuloma formation
- PMID: 23444049
- PMCID: PMC3580335
- DOI: 10.3389/fimmu.2013.00043
Chemokines in innate and adaptive granuloma formation
Abstract
Granulomas are cellular inflammations that vary widely in histologic appearance depending upon the inciting agent and immunologic status of the responding host. Despite their heterogeneity, granulomas are at their core an ancient innate sequestration response characterized by the accumulation of mononuclear phagocytes. In fact, this innate cellular response was first observed by Metchnikov in simple invertebrates. Among higher vertebrates, environmental pressures have resulted in the evolution of more sophisticated adaptive immune responses which can be superimposed upon and modify the character of granulomatous inflammation. Compared to immune responses that rapidly neutralize and eliminate infectious agents, the granuloma represents a less desirable "fall back" response which still has value to the host but can be co-opted by certain infectious agents and contribute to bystander organ damage. Understanding granulomas requires an analysis of the complex interplay of innate and adaptive molecular signals that govern the focal accumulation and activity of their cellular components. Among these signals, small molecular weight chemoattractant proteins known as chemokines are potentially important contributors as they participate in both directing leukocyte migration and function. This tract will discuss the contribution of chemokines to the development of innate and adaptive granuloma formation, as well as describe their relationship to more recently evolved cytokines generated during adaptive immune responses.
Keywords: chemokine receptors; chemokines; granuloma; inflammation; innate immunity.
Figures
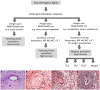
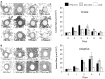
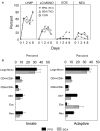

References
-
- Chensue S. W., Warmington K., Ruth J., Lincoln P., Kuo M. C., Kunkel S. L. (1994). Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am. J. Pathol. 145, 1105–1113 - PMC - PubMed
-
- Chensue S. W., Warmington K. S., Allenspach E. J., Lu B., Gerard C., Kunkel S. L., et al. (1999). Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J. Immunol. 163, 165–173 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

