Characterization of human vocal fold fibroblasts derived from chronic scar
- PMID: 23444190
- PMCID: PMC3584344
- DOI: 10.1002/lary.23681
Characterization of human vocal fold fibroblasts derived from chronic scar
Abstract
Objectives/hypothesis: In vitro modeling of cell-matrix interactions that occur during human vocal fold scarring is uncommon, as primary human vocal fold scar fibroblast cell lines are difficult to acquire. The purpose of this study was to characterize morphologic features, growth kinetics, contractile properties, α-smooth muscle actin (α-SMA) protein expression and gene expression profile of human vocal fold fibroblasts derived from scar (sVFF) relative to normal vocal fold fibroblasts (nVFF).
Study design: In vitro.
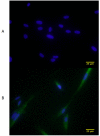
Methods: We successfully cultured human vocal fold fibroblasts from tissue explants of scarred vocal folds from a 56-year-old female and compared these to normal fibroblasts from a 59-year-old female. Growth and proliferation were assessed by daily cell counts, and morphology was compared at 60% confluence for 5 days. Gel contraction assays were evaluated after seeding cells within a collagen matrix. α-SMA was measured using western blotting and immunocytochemistry (ICC). Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) was used to assess differential extracellular matrix gene expression between the two cell types.
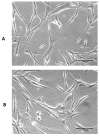
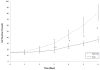
Results: sVFF were morphologically indistinguishable from nVFF. sVFF maintained significantly lower proliferation rates relative to nVFF on days 3 to 6 (day 3: P = .0138; days 4, 5, and 6: P < .0001). There were no significant differences in contractile properties between the two cell types at any time point (0 hours: P = .70, 24 hours: P = .79, 48 hours: P = .58). ICC and western blot analyses revealed increased expression of α-SMA in sVFF as compared with nVFF at passages 4 and 5, but not at passage 6 (passage 4: P = .006, passage 5: P = .0015, passage 6: P = .8860). Analysis of 84 extracellular matrix genes using qRT-PCR revealed differential expression of 15 genes (P < .01).
Conclusions: nVFF and sVFF displayed differences in proliferation rates, α-SMA expression, and gene expression, whereas no differences were observed in contractile properties or morphology. Further investigation with a larger sample size is necessary to confirm these findings.
Copyright © 2013 The American Laryngological, Rhinological, and Otological Society, Inc.
Figures


References
-
- Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. - PubMed
-
- Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg. 2005;13:143–147. - PubMed
-
- Hirano S, Minamiguchi S, Yamashita M, Ohno T, Kanemaru S-I, Kitamura M. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. - PubMed
-
- Benninger MS, Alessi D, Archer S, et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996;115:474–482. - PubMed
-
- Gray SD. Cellular physiology of the vocal folds. Otolaryngologic clinics of North America. 2000;33:679–698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

