Resolution of defective dorsal aortae patterning in Sema3E-deficient mice occurs via angiogenic remodeling
- PMID: 23444297
- PMCID: PMC4374655
- DOI: 10.1002/dvdy.23949
Resolution of defective dorsal aortae patterning in Sema3E-deficient mice occurs via angiogenic remodeling
Abstract
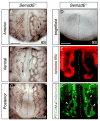
Background: Neuronal guidance cues influence endothelial cell (EC) behavior to shape the embryonic vascular system. The repulsive neuronal guidance cue, Semaphorin 3E (Sema3E), is critical for creating avascular zones that instruct and subsequently pattern the first embryonic vessels, the paired dorsal aortae (DA). Sema3E(-) (/) (-) embryos develop highly branched plexus-like vessels during vasculogenesis, instead of smooth paired vessels. Unexpectedly, despite these severe DA patterning defects, mutant mice are viable throughout adulthood.
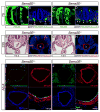
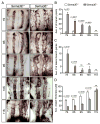
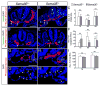
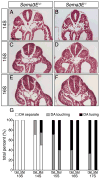
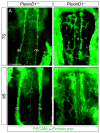
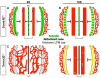
Results: Examination of Sema3E(-) (/) (-) mice reveals that the plexus-like DA resolve into single, unbranched vessels between embryonic day (E) E8.25 and E8.75. Although fusion of Sema3E(-) (/) (-) DA occurs slightly earlier than in heterozygotes, the DA are otherwise indistinguishable, suggesting a complete "rescue" in their development. Resolution of the DA null plexuses occurs by remodeling rather than by means of changes in cell proliferation or death.
Conclusions: Normalization of Sema3E(-) (/) (-) DA patterning defects demonstrates resilience of embryonic vascular patterning programs. Additional repulsive guidance cues within the lateral plate mesoderm likely re-establish avascular zones lost in Sema3E(-) (/) (-) embryos and guide resolution of mutant plexus into branchless, parallel aortae. Our observations explain how Sema3E(-) (/) (-) mice survive throughout development and into adulthood, despite severe initial vascular defects.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
Similar articles
-
Integration of repulsive guidance cues generates avascular zones that shape mammalian blood vessels.Circ Res. 2012 Jan 6;110(1):34-46. doi: 10.1161/CIRCRESAHA.111.249847. Epub 2011 Nov 10. Circ Res. 2012. PMID: 22076636 Free PMC article.
-
Semaphorin 3d and semaphorin 3e direct endothelial motility through distinct molecular signaling pathways.J Biol Chem. 2014 Jun 27;289(26):17971-9. doi: 10.1074/jbc.M113.544833. Epub 2014 May 13. J Biol Chem. 2014. PMID: 24825896 Free PMC article.
-
Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism.Genes Dev. 2011 Jul 1;25(13):1399-411. doi: 10.1101/gad.2042011. Genes Dev. 2011. PMID: 21724832 Free PMC article.
-
Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
-
Dorsal aorta formation: separate origins, lateral-to-medial migration, and remodeling.Dev Growth Differ. 2013 Jan;55(1):113-29. doi: 10.1111/dgd.12010. Epub 2012 Nov 8. Dev Growth Differ. 2013. PMID: 23294360 Review.
Cited by
-
Class 3 semaphorins in cardiovascular development.Cell Adh Migr. 2016 Nov;10(6):641-651. doi: 10.1080/19336918.2016.1212805. Epub 2016 Jul 20. Cell Adh Migr. 2016. PMID: 27439112 Free PMC article. Review.
-
Blood vessel crosstalk during organogenesis-focus on pancreas and endothelial cells.Wiley Interdiscip Rev Dev Biol. 2016 Sep;5(5):598-617. doi: 10.1002/wdev.240. Epub 2016 Jun 21. Wiley Interdiscip Rev Dev Biol. 2016. PMID: 27328421 Free PMC article. Review.
-
WNK1 collaborates with TGF-β in endothelial cell junction turnover and angiogenesis.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2203743119. doi: 10.1073/pnas.2203743119. Epub 2022 Jul 22. Proc Natl Acad Sci U S A. 2022. PMID: 35867836 Free PMC article.
-
Neural crest-specific deletion of Rbfox2 in mice leads to craniofacial abnormalities including cleft palate.Elife. 2019 Jun 26;8:e45418. doi: 10.7554/eLife.45418. Elife. 2019. PMID: 31241461 Free PMC article.
-
View Point: Semaphorin-3E: An Emerging Modulator of Natural Killer Cell Functions?Int J Mol Sci. 2017 Nov 5;18(11):2337. doi: 10.3390/ijms18112337. Int J Mol Sci. 2017. PMID: 29113093 Free PMC article. Review.
References
-
- Coffin JD, Harrison J, Schwartz S, Heimark R. Angioblast differentiation and morphogenesis of the vascular endothelium in the mouse embryo. Developmental biology. 1991;148:51–62. - PubMed
-
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. - PubMed
-
- Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19:1013–1021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

