Progressive transverse microtubule array organization in hormone-induced Arabidopsis hypocotyl cells
- PMID: 23444330
- PMCID: PMC3608785
- DOI: 10.1105/tpc.112.107326
Progressive transverse microtubule array organization in hormone-induced Arabidopsis hypocotyl cells
Abstract
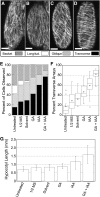
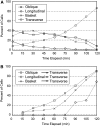
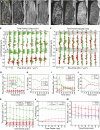
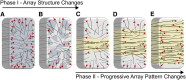
The acentriolar cortical microtubule arrays in dark-grown hypocotyl cells organize into a transverse coaligned pattern that is critical for axial plant growth. In light-grown Arabidopsis thaliana seedlings, the cortical array on the outer (periclinal) cell face creates a variety of array patterns with a significant bias (>3:1) for microtubules polymerizing edge-ward and into the side (anticlinal) faces of the cell. To study the mechanisms required for creating the transverse coalignment, we developed a dual-hormone protocol that synchronously induces ∼80% of the light-grown hypocotyl cells to form transverse arrays over a 2-h period. Repatterning occurred in two phases, beginning with an initial 30 to 40% decrease in polymerizing plus ends prior to visible changes in the array pattern. Transverse organization initiated at the cell's midzone by 45 min after induction and progressed bidirectionally toward the apical and basal ends of the cell. Reorganization corrected the edge-ward bias in polymerization and proceeded without transiting through an obligate intermediate pattern. Quantitative comparisons of uninduced and induced microtubule arrays showed a limited deconstruction of the initial periclinal array followed by a progressive array reorganization to transverse coordinated between the anticlinal and periclinal cell faces.
Figures


Similar articles
-
A Cycloheximide-Sensitive Step in Transverse Microtubule Array Patterning.Plant Physiol. 2018 Oct;178(2):684-698. doi: 10.1104/pp.18.00672. Epub 2018 Aug 28. Plant Physiol. 2018. PMID: 30154175 Free PMC article.
-
Microtubule Array Patterns Have a Common Underlying Architecture in Hypocotyl Cells.Plant Physiol. 2018 Jan;176(1):307-325. doi: 10.1104/pp.17.01112. Epub 2017 Sep 11. Plant Physiol. 2018. PMID: 28894021 Free PMC article.
-
Exogenous Auxin Induces Transverse Microtubule Arrays Through TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX Receptors.Plant Physiol. 2020 Feb;182(2):892-907. doi: 10.1104/pp.19.00928. Epub 2019 Nov 25. Plant Physiol. 2020. PMID: 31767691 Free PMC article.
-
Reorganization of the plant cortical microtubule array.Curr Opin Plant Biol. 2013 Dec;16(6):693-7. doi: 10.1016/j.pbi.2013.09.006. Curr Opin Plant Biol. 2013. PMID: 24446545 Review.
-
Cortical microtubule arrays in the Arabidopsis seedling.Curr Opin Plant Biol. 2008 Feb;11(1):94-8. doi: 10.1016/j.pbi.2007.12.001. Epub 2008 Jan 15. Curr Opin Plant Biol. 2008. PMID: 18226578 Review.
Cited by
-
Microtubules in plants.Arabidopsis Book. 2015 Apr 27;13:e0179. doi: 10.1199/tab.0179. eCollection 2015. Arabidopsis Book. 2015. PMID: 26019693 Free PMC article.
-
Abscisic acid induces ectopic outgrowth in epidermal cells through cortical microtubule reorganization in Arabidopsis thaliana.Sci Rep. 2015 Jun 12;5:11364. doi: 10.1038/srep11364. Sci Rep. 2015. PMID: 26068445 Free PMC article.
-
Phytohormone cytokinin guides microtubule dynamics during cell progression from proliferative to differentiated stage.EMBO J. 2020 Sep 1;39(17):e104238. doi: 10.15252/embj.2019104238. Epub 2020 Jul 15. EMBO J. 2020. PMID: 32667089 Free PMC article.
-
A transient radial cortical microtubule array primes cell division in Arabidopsis.Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2320470121. doi: 10.1073/pnas.2320470121. Epub 2024 Jul 11. Proc Natl Acad Sci U S A. 2024. PMID: 38990951 Free PMC article.
-
Arabidopsis IPGA1 is a microtubule-associated protein essential for cell expansion during petal morphogenesis.J Exp Bot. 2019 Oct 15;70(19):5231-5243. doi: 10.1093/jxb/erz284. J Exp Bot. 2019. PMID: 31198941 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

