A membrane-associated β-catenin/Oct4 complex correlates with ground-state pluripotency in mouse embryonic stem cells
- PMID: 23444350
- PMCID: PMC3585656
- DOI: 10.1242/dev.085654
A membrane-associated β-catenin/Oct4 complex correlates with ground-state pluripotency in mouse embryonic stem cells
Abstract
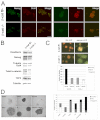
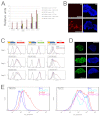
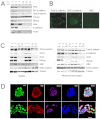
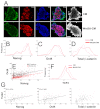
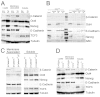
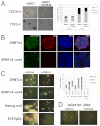
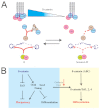
The maintenance of pluripotency in mouse embryonic stem cells (mESCs) relies on the activity of a transcriptional network that is fuelled by the activity of three transcription factors (Nanog, Oct4 and Sox2) and balanced by the repressive activity of Tcf3. Extracellular signals modulate the activity of the network and regulate the differentiation capacity of the cells. Wnt/β-catenin signaling has emerged as a significant potentiator of pluripotency: increases in the levels of β-catenin regulate the activity of Oct4 and Nanog, and enhance pluripotency. A recent report shows that β-catenin achieves some of these effects by modulating the activity of Tcf3, and that this effect does not require its transcriptional activation domain. Here, we show that during self-renewal there is negligible transcriptional activity of β-catenin and that this is due to its tight association with membranes, where we find it in a complex with Oct4 and E-cadherin. Differentiation triggers a burst of Wnt/β-catenin transcriptional activity that coincides with the disassembly of the complex. Our results establish that β-catenin, but not its transcriptional activity, is central to pluripotency acting through a β-catenin/Oct4 complex.
Figures

References
-
- Aiba K., Sharov A. A., Carter M. G., Foroni C., Vescovi A. L., Ko M. S. (2006). Defining a developmental path to neural fate by global expression profiling of mouse embryonic stem cells and adult neural stem/progenitor cells. Stem Cells 24, 889–895 - PubMed
-
- Angers S., Moon R. T. (2009). Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 - PubMed
-
- Anton R., Kestler H. A., Kühl M. (2007). Beta-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 581, 5247–5254 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD052115/HD/NICHD NIH HHS/United States
- BB/D526088/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 079249/Wellcome Trust/United Kingdom
- 1R21HD057525-01/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R21 HD057525/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

