HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment
- PMID: 23444397
- PMCID: PMC3640201
- DOI: 10.1093/eurheartj/eht055
HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment
Abstract
Aims: Niacin has potentially favourable effects on lipids, but its effect on cardiovascular outcomes is uncertain. HPS2-THRIVE is a large randomized trial assessing the effects of extended release (ER) niacin in patients at high risk of vascular events.
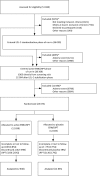
Methods and results: Prior to randomization, 42 424 patients with occlusive arterial disease were given simvastatin 40 mg plus, if required, ezetimibe 10 mg daily to standardize their low-density lipoprotein (LDL)-lowering therapy. The ability to remain compliant with ER niacin 2 g plus laropiprant 40 mg daily (ERN/LRPT) for ~1 month was then assessed in 38 369 patients and about one-third were excluded (mainly due to niacin side effects). A total of 25 673 patients were randomized between ERN/LRPT daily vs. placebo and were followed for a median of 3.9 years. By the end of the study, 25% of participants allocated ERN/LRPT vs. 17% allocated placebo had stopped their study treatment. The most common medical reasons for stopping ERN/LRPT were related to skin, gastrointestinal, diabetes, and musculoskeletal side effects. When added to statin-based LDL-lowering therapy, allocation to ERN/LRPT increased the risk of definite myopathy [75 (0.16%/year) vs. 17 (0.04%/year): risk ratio 4.4; 95% CI 2.6-7.5; P < 0.0001]; 7 vs. 5 were rhabdomyolysis. Any myopathy (definite or incipient) was more common among participants in China [138 (0.66%/year) vs. 27 (0.13%/year)] than among those in Europe [17 (0.07%/year) vs. 11 (0.04%/year)]. Consecutive alanine transaminase >3× upper limit of normal, in the absence of muscle damage, was seen in 48 (0.10%/year) ERN/LRPT vs. 30 (0.06%/year) placebo allocated participants.
Conclusion: The risk of myopathy was increased by adding ERN/LRPT to simvastatin 40 mg daily (with or without ezetimibe), particularly in Chinese patients whose myopathy rates on simvastatin were higher. Despite the side effects of ERN/LRPT, among individuals who were able to tolerate it for ~1 month, three-quarters continued to take it for ~4 years.
Keywords: ER niacin/laropiprant; HDL-cholesterol; LDL-cholesterol; cardiovascular disease; secondary prevention.
Figures

Comment in
-
The difficult search for a 'partner' of statins in lipid-targeted prevention of vascular events: the re-emergence and fall of niacin.Eur Heart J. 2013 May;34(17):1254-7. doi: 10.1093/eurheartj/eht064. Epub 2013 Feb 26. Eur Heart J. 2013. PMID: 23444398 No abstract available.
-
When clinical trials fail to address treatment gaps: the failure of niacin-laropiprant to reduce cardiovascular events.Curr Atheroscler Rep. 2013 Jun;15(6):332. doi: 10.1007/s11883-013-0332-x. Curr Atheroscler Rep. 2013. PMID: 23605289 No abstract available.
-
Niacin's effect on cardiovascular risk: have we finally learned our lesson?Cleve Clin J Med. 2014 May;81(5):275-7. doi: 10.3949/ccjm.81a.13067. Cleve Clin J Med. 2014. PMID: 24789584 No abstract available.
References
-
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2012;33:1635–1701. - PubMed
-
- Bennet A, Di Angelantonio E, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, Rumley A, Lowe GDO, Danesh J, Gudnason V. lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med. 2008;168:598–608. - PubMed
-
- Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005;258:94–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

