Mi2β-mediated silencing of the fetal γ-globin gene in adult erythroid cells
- PMID: 23444401
- PMCID: PMC3637018
- DOI: 10.1182/blood-2012-11-466227
Mi2β-mediated silencing of the fetal γ-globin gene in adult erythroid cells
Abstract
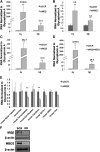
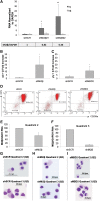
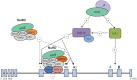
An understanding of the human fetal to adult hemoglobin switch offers the potential to ameliorate β-type globin gene disorders such as sickle cell anemia and β-thalassemia through activation of the fetal γ-globin gene. Chromatin modifying complexes, including MBD2-NuRD and GATA-1/FOG-1/NuRD, play a role in γ-globin gene silencing, and Mi2β (CHD4) is a critical component of NuRD complexes. We observed that knockdown of Mi2β relieves γ-globin gene silencing in β-YAC transgenic murine chemical inducer of dimerization hematopoietic cells and in CD34(+) progenitor-derived human primary adult erythroid cells. We show that independent of MBD2-NuRD and GATA-1/FOG-1/NuRD, Mi2β binds directly to and positively regulates both the KLF1 and BCL11A genes, which encode transcription factors critical for γ-globin gene silencing during β-type globin gene switching. Remarkably, <50% knockdown of Mi2β is sufficient to significantly induce γ-globin gene expression without disrupting erythroid differentiation of primary human CD34(+) progenitors. These results indicate that Mi2β is a potential target for therapeutic induction of fetal hemoglobin.
Figures




References
-
- Thein SL, Menzel S. Discovering the genetics underlying foetal haemoglobin production in adults. Br J Haematol. 2009;145(4):455–467. - PubMed
-
- Peschle C, Mavilio F, Carè A, et al. Haemoglobin switching in human embryos: asynchrony of zeta—alpha and epsilon—gamma-globin switches in primitive and definite erythropoietic lineage. Nature. 1985;313(5999):235–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

