Effect of telehealth on quality of life and psychological outcomes over 12 months (Whole Systems Demonstrator telehealth questionnaire study): nested study of patient reported outcomes in a pragmatic, cluster randomised controlled trial
- PMID: 23444424
- PMCID: PMC3582704
- DOI: 10.1136/bmj.f653
Effect of telehealth on quality of life and psychological outcomes over 12 months (Whole Systems Demonstrator telehealth questionnaire study): nested study of patient reported outcomes in a pragmatic, cluster randomised controlled trial
Abstract
Objective: To assess the effect of second generation, home based telehealth on health related quality of life, anxiety, and depressive symptoms over 12 months in patients with long term conditions.
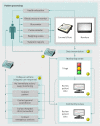
Design: A study of patient reported outcomes (the Whole Systems Demonstrator telehealth questionnaire study; baseline n=1573) was nested in a pragmatic, cluster randomised trial of telehealth (the Whole Systems Demonstrator telehealth trial, n=3230). General practice was the unit of randomisation, and telehealth was compared with usual care. Data were collected at baseline, four months (short term), and 12 months (long term). Primary intention to treat analyses tested treatment effectiveness; multilevel models controlled for clustering by general practice and a range of covariates. Analyses were conducted for 759 participants who completed questionnaire measures at all three time points (complete case cohort) and 1201 who completed the baseline assessment plus at least one other assessment (available case cohort). Secondary per protocol analyses tested treatment efficacy and included 633 and 1108 participants in the complete case and available case cohorts, respectively.
Setting: Provision of primary and secondary care via general practices, specialist nurses, and hospital clinics in three diverse regions of England (Cornwall, Kent, and Newham), with established integrated health and social care systems.
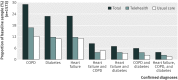
Participants: Patients with chronic obstructive pulmonary disease (COPD), diabetes, or heart failure recruited between May 2008 and December 2009.
Main outcome measures: Generic, health related quality of life (assessed by physical and mental health component scores of the SF-12, and the EQ-5D), anxiety (assessed by the six item Brief State-Trait Anxiety Inventory), and depressive symptoms (assessed by the 10 item Centre for Epidemiological Studies Depression Scale).
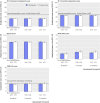
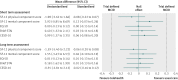
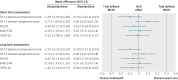
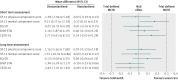
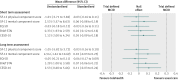
Results: In the intention to treat analyses, differences between treatment groups were small and non-significant for all outcomes in the complete case (0.480 ≤ P ≤ 0.904) or available case (0.181 ≤ P ≤ 0.905) cohorts. The magnitude of differences between trial arms did not reach the trial defined, minimal clinically important difference (0.3 standardised mean difference) for any outcome in either cohort at four or 12 months. Per protocol analyses replicated the primary analyses; the main effect of trial arm (telehealth v usual care) was non-significant for any outcome (complete case cohort 0.273 ≤ P ≤ 0.761; available case cohort 0.145 ≤ P ≤ 0.696).
Conclusions: Second generation, home based telehealth as implemented in the Whole Systems Demonstrator Evaluation was not effective or efficacious compared with usual care only. Telehealth did not improve quality of life or psychological outcomes for patients with chronic obstructive pulmonary disease, diabetes, or heart failure over 12 months. The findings suggest that concerns about potentially deleterious effect of telehealth are unfounded for most patients.
Trial registration: ISRCTN43002091.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

References
-
- Office for National Statistics. The interactive population pyramid. 2011. www.neighbourhood.statistics.gov.uk/HTMLDocs/dvc1/UKPyramid.html.
-
- Spillman BC, Lubitz J. The effect of longevity on spending for acute and long-term care. N Engl J Med 2000;342:1409-15. - PubMed
-
- Robine J-M, Romieu I, Michel J. Trends in health expectancies. In: Robine J-M, Jagger C, Mathers C, Crimmins EM, Suzman R, eds. Determining health expectancies. John Wiley and Sons, 2003:75-104.
-
- Doblhammer G, Kytir J. Compression or expansion of morbidity? Trends in healthy-life expectancy in the elderly Austrian population between 1978 and 1998. Soc Sci Med 2001;52:385-91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
