Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis
- PMID: 23444845
- PMCID: PMC3822649
- DOI: 10.1111/jcmm.12028
Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis
Abstract
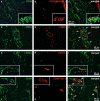
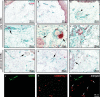
Telocytes, a peculiar type of stromal cells, have been recently identified in a variety of tissues and organs, including human skin. Systemic sclerosis (SSc, scleroderma) is a complex connective tissue disease characterized by fibrosis of the skin and internal organs. We presently investigated telocyte distribution and features in the skin of SSc patients compared with normal skin. By an integrated immunohistochemical and transmission electron microscopy approach, we confirmed that telocytes were present in human dermis, where they were mainly recognizable by their typical ultrastructural features and were immunophenotypically characterized by CD34 expression. Our findings also showed that dermal telocytes were immunophenotypically negative for CD31/PECAM-1 (endothelial cells), α-SMA (myofibroblasts, pericytes, vascular smooth muscle cells), CD11c (dendritic cells, macrophages), CD90/Thy-1 (fibroblasts) and c-kit/CD117 (mast cells). In normal skin, telocytes were organized to form three-dimensional networks distributed among collagen bundles and elastic fibres, and surrounded microvessels, nerves and skin adnexa (hair follicles, sebaceous and sweat glands). Telocytes displayed severe ultrastructural damages (swollen mitochondria, cytoplasmic vacuolization, lipofuscinic bodies) suggestive of ischaemia-induced cell degeneration and were progressively lost from the clinically affected skin of SSc patients. Telocyte damage and loss evolved differently according to SSc subsets and stages, being more rapid and severe in diffuse SSc. Briefly, in human skin telocytes are a distinct stromal cell population. In SSc skin, the progressive loss of telocytes might (i) contribute to the altered three-dimensional organization of the extracellular matrix, (ii) reduce the control of fibroblast, myofibroblast and mast cell activity, and (iii) impair skin regeneration and/or repair.
© 2013 The Authors. Published by Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures







References
-
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. - PubMed
-
- Jain S, Shahane A, Derk CT. Interstitial lung disease in systemic sclerosis: pathophysiology, current and new advances in therapy. Inflamm Allergy Drug Targets. 2012;11:266–77. - PubMed
-
- Manetti M, Neumann E, Milia AF, et al. Severe fibrosis and increased expression of fibrogenic cytokines in the gastric wall of systemic sclerosis patients. Arthritis Rheum. 2007;56:3442–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

