Synthesis and biological evaluation of 2-(alkoxycarbonyl)-3-anilinobenzo[b]thiophenes and thieno[2,3-b]pyridines as new potent anticancer agents
- PMID: 23445496
- PMCID: PMC3646584
- DOI: 10.1021/jm400043d
Synthesis and biological evaluation of 2-(alkoxycarbonyl)-3-anilinobenzo[b]thiophenes and thieno[2,3-b]pyridines as new potent anticancer agents
Abstract
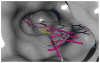
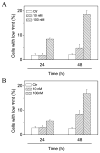
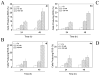
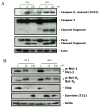
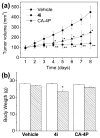
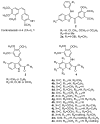
Two new series of inhibitors of tubulin polymerization based on the 2-(alkoxycarbonyl)-3-(3',4',5'-trimethoxyanilino)benzo[b]thiophene and thieno[2,3-b]pyridine molecular skeletons were synthesized and evaluated for antiproliferative activity on a panel of cancer cell lines, inhibition of tubulin polymerization, cell cycle effects, and in vivo potency. Antiproliferative activity was strongly dependent on the position of the methyl group on the benzene portion of the benzo[b]thiophene nucleus, with the greatest activity observed when the methyl was located at the C-6 position. Also, in the smaller thieno[2,3-b]pyridine series, the introduction of the methyl group at the C-6 position resulted in improvement of antiproliferative activity to the nanomolar level. The most active compounds (4i and 4n) did not induce cell death in normal human lymphocytes, suggesting that the compounds may be selective against cancer cells. Compound 4i significantly inhibited in vivo the growth of a syngeneic hepatocellular carcinoma in Balb/c mice.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Amos LA. Microtubule structure and its stabilisation. Org Biomol Chem. 2004;2:2153–2160. - PubMed
-
- Walczak CE. Microtubule dynamics and tubulin interacting proteins. Curr Opin Cell Biol. 2000;12:52–56. - PubMed
-
- Perez EA. Microtubule inhibitors: differentiating tubulin inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther. 2009;8:2086–2095. - PubMed
-
- Carlson RO. New tubulin targeting agents currently in clinical development. Expert Opin Invest Drugs. 2008;17:707–722. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

