PET/MR: a paradigm shift
- PMID: 23446110
- PMCID: PMC3584300
- DOI: 10.1102/1470-7330.2013.0005
PET/MR: a paradigm shift
Abstract
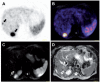
More than a decade ago, multimodality imaging was introduced into clinical routine with the development of the positron emission tomography (PET)/computed tomography (CT) technique. Since then, PET/CT has been widely accepted in clinical imaging and has emerged as one of the main cancer imaging modalities. With the recent development of combined PET/magnetic resonance (MR) systems for clinical use, a promising new hybrid imaging modality is now becoming increasingly available. The combination of functional information delivered by PET with the morphologic and functional imaging of MR imaging (e.g., diffusion-weighted imaging, dynamic contrast-enhanced MR imaging and MR spectroscopy) offers exciting possibilities for clinical applications as well as basic research. However, the differences between CT and MR imaging are fundamental. This also leads to distinct differences between PET/CT and PET/MR not only regarding image interpretation but also concerning data acquisition, data processing and image reconstruction. This article provides an overview of the principal differences between PET/CT and PET/MR in terms of scanner design and technology, attenuation correction, speed, acquisition protocols, radiation exposure and safety aspects. PET/MR is expected to show advantages over PET/CT in clinical applications in which MR is known to be superior to CT due to its high intrinsic soft tissue contrast. However, as of now, only assumptions can be made about the future clinical role of PET/MR, as data about the performance of PET/MR in the clinical setting are still limited. The possible future clinical use of PET/MR in oncology, neurology and neurooncology, cardiology and imaging of inflammation is discussed.
Figures









References
-
- Beyer T, Townsend DW, Brun T, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. PMid:10945530. - PubMed
-
- Czernin J, Allen-Auerbach M, Schelbert HR. Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med. 2007;48(Suppl 1):78S–88S. PMid:17204723. - PubMed
-
- Picchio M, Mansueto M, Crivellaro C, et al. PET/CT and contrast enhanced CT in single vs. two separate sessions: a cost analysis study. Q J Nucl Med Mol Imaging. 2012;56:309–316. PMid:22510702. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
