Disruption of a spermatogenic cell-specific mouse enolase 4 (eno4) gene causes sperm structural defects and male infertility
- PMID: 23446454
- PMCID: PMC4013874
- DOI: 10.1095/biolreprod.112.107128
Disruption of a spermatogenic cell-specific mouse enolase 4 (eno4) gene causes sperm structural defects and male infertility
Abstract
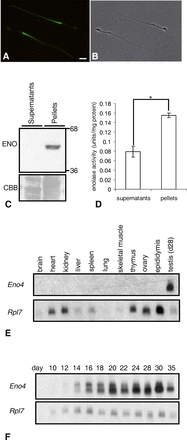
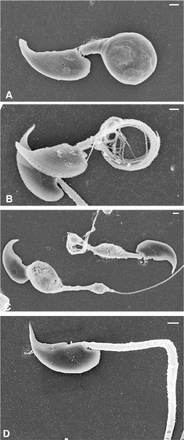
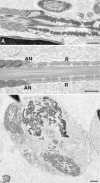
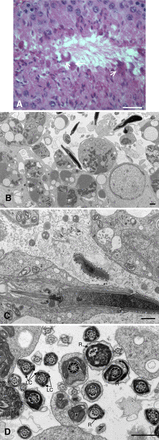
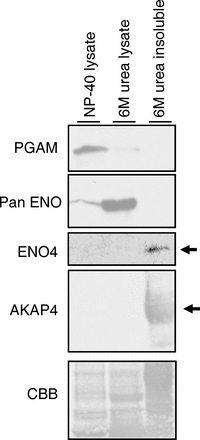
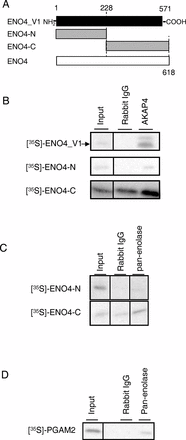
Sperm utilize glycolysis to generate ATP required for motility, and several spermatogenic cell-specific glycolytic isozymes are associated with the fibrous sheath (FS) in the principal piece of the sperm flagellum. We used proteomics and molecular biology approaches to confirm earlier reports that a novel enolase is present in mouse sperm. We then found that a pan-enolase antibody, but not antibodies to ENO2 and ENO3, recognized a protein in the principal piece of the mouse sperm flagellum. Database analyses identified two previously uncharacterized enolase family-like candidate genes, 64306537H0Rik and Gm5506. Northern analysis indicated that 64306537H0Rik (renamed Eno4) was transcribed in testes of mice by Postnatal Day 12. To determine the role of ENO4, we generated mice using embryonic stem cells in which an Eno4 allele was disrupted by a gene trap containing a beta galactosidase (beta-gal) reporter (Eno4(+/Gt)). Expression of beta-gal occurred in the testis, and male mice homozygous for the gene trap allele (Eno4(Gt/Gt)) were infertile. Epididymal sperm numbers were 2-fold lower and sperm motility was reduced substantially in Eno4(Gt/Gt) mice compared to wild-type mice. Sperm from Eno4(Gt/Gt) mice had a coiled flagellum and a disorganized FS. The Gm5506 gene encodes a protein identical to ENO1 and also is transcribed at a low level in testis. We conclude that ENO4 is required for normal assembly of the FS and provides most of the enolase activity in sperm and that Eno1 and/or Gm5506 may encode a minor portion of the enolase activity in sperm.
Figures



References
-
- Mori C, Nakamura N, Welch JE, Gotoh H, Goulding EH, Fujioka M, Eddy EM. Mouse spermatogenic cell-specific type 1 hexokinase (mHk1-s) transcripts are expressed by alternative splicing from the mHk1 gene and the HK1-S protein is localized mainly in the sperm tail. Mol Reprod Dev 1998; 49: 374–385. - PubMed
-
- Vemuganti SA, Bell TA, Scarlett DO, Parker CE, Pardo-Manuel de Villena F, O'Brien DA. Three male germline-specific aldolase A isozymes are generated by alternative splicing and retrotransposition. Dev Biol 2007; 309: 18–31. - PubMed
-
- McCarrey JR, Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature 1987; 326: 501–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

