Effect of the levonorgestrel intrauterine device on genital HIV-1 RNA shedding among HIV-1-infected women not taking antiretroviral therapy in Nairobi, Kenya
- PMID: 23446496
- PMCID: PMC3668353
- DOI: 10.1097/QAI.0b013e31828decf8
Effect of the levonorgestrel intrauterine device on genital HIV-1 RNA shedding among HIV-1-infected women not taking antiretroviral therapy in Nairobi, Kenya
Abstract
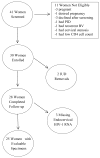
The effect of the levonorgestrel-releasing intrauterine device (LNG-IUD) on genital HIV-1 RNA shedding and inflammation among 25 HIV-infected women was evaluated. Blood, endocervical, and cervicovaginal lavage samples were collected from HIV-infected women not taking antiretrovirals before LNG-IUD insertion and 1 month, 3 month, and 6 months thereafter. HIV-1 RNA was quantitated by real-time reverse transcriptase-polymerase chain reaction. Inflammatory markers were measured by enzyme immunoassay. Genital HIV-1 RNA shedding and inflammatory markers did not differ between LNG-IUD placement and month 6, with the exception of interleukin 1β that increased (0.42 log10; 95% confidence interval: 0.10 to 0.75). The LNG-IUD did not increase genital HIV-1 RNA shedding after 6 months of use.
Conflict of interest statement
Conflict of Interest: No conflicts declared.
References
-
- World Health Organization. PMTCT strategic vision 2010–2015: prevention mother-to-child transmission of HIV to reach the UNGASS and Milennium Development Goals. Vol. 2010 Switzerland: 2010. Feb 2,
-
- Luukkainen T, Toivonen J. Levonorgestrel-releasing IUD as a method of contraception with therapeutic properties. Contraception. 1995;52:269–76. - PubMed
-
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56–72. - PubMed
-
- Kaunitz AM, Meredith S, Inki P, Kubba A, Sanchez-Ramos L. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104–16. - PubMed
-
- World Health Organization. Medical eligibility criteria for contraceptive use. Switzerland: 2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources