Neural integrators for decision making: a favorable tradeoff between robustness and sensitivity
- PMID: 23446688
- PMCID: PMC3653050
- DOI: 10.1152/jn.00976.2012
Neural integrators for decision making: a favorable tradeoff between robustness and sensitivity
Abstract
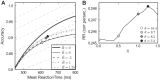
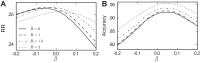
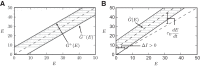
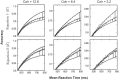
A key step in many perceptual decision tasks is the integration of sensory inputs over time, but a fundamental questions remain about how this is accomplished in neural circuits. One possibility is to balance decay modes of membranes and synapses with recurrent excitation. To allow integration over long timescales, however, this balance must be exceedingly precise. The need for fine tuning can be overcome via a "robust integrator" mechanism in which momentary inputs must be above a preset limit to be registered by the circuit. The degree of this limiting embodies a tradeoff between sensitivity to the input stream and robustness against parameter mistuning. Here, we analyze the consequences of this tradeoff for decision-making performance. For concreteness, we focus on the well-studied random dot motion discrimination task and constrain stimulus parameters by experimental data. We show that mistuning feedback in an integrator circuit decreases decision performance but that the robust integrator mechanism can limit this loss. Intriguingly, even for perfectly tuned circuits with no immediate need for a robustness mechanism, including one often does not impose a substantial penalty for decision-making performance. The implication is that robust integrators may be well suited to subserve the basic function of evidence integration in many cognitive tasks. We develop these ideas using simulations of coupled neural units and the mathematics of sequential analysis.
Keywords: decision making; neural integrator.
Figures















Similar articles
-
Dynamic afferent synapses to decision-making networks improve performance in tasks requiring stimulus associations and discriminations.J Neurophysiol. 2012 Jul;108(2):513-27. doi: 10.1152/jn.00806.2011. Epub 2012 Mar 28. J Neurophysiol. 2012. PMID: 22457467 Free PMC article.
-
Robustness of learning that is based on covariance-driven synaptic plasticity.PLoS Comput Biol. 2008 Mar 7;4(3):e1000007. doi: 10.1371/journal.pcbi.1000007. PLoS Comput Biol. 2008. PMID: 18369414 Free PMC article.
-
Same or different? A neural circuit mechanism of similarity-based pattern match decision making.J Neurosci. 2011 May 11;31(19):6982-96. doi: 10.1523/JNEUROSCI.6150-10.2011. J Neurosci. 2011. PMID: 21562260 Free PMC article.
-
Single-trial analysis of neuroimaging data: inferring neural networks underlying perceptual decision-making in the human brain.IEEE Rev Biomed Eng. 2009;2:97-109. doi: 10.1109/RBME.2009.2034535. IEEE Rev Biomed Eng. 2009. PMID: 22275042 Free PMC article. Review.
-
Neural processing as causal inference.Curr Opin Neurobiol. 2011 Oct;21(5):774-81. doi: 10.1016/j.conb.2011.05.018. Curr Opin Neurobiol. 2011. PMID: 21742484 Review.
Cited by
-
Mechanisms of Persistent Activity in Cortical Circuits: Possible Neural Substrates for Working Memory.Annu Rev Neurosci. 2017 Jul 25;40:603-627. doi: 10.1146/annurev-neuro-070815-014006. Annu Rev Neurosci. 2017. PMID: 28772102 Free PMC article. Review.
-
A dynamic neural field model of continuous input integration.Biol Cybern. 2021 Oct;115(5):451-471. doi: 10.1007/s00422-021-00893-7. Epub 2021 Aug 21. Biol Cybern. 2021. PMID: 34417880
-
Neuronal pattern separation of motion-relevant input in LIP activity.J Neurophysiol. 2017 Feb 1;117(2):738-755. doi: 10.1152/jn.00145.2016. Epub 2016 Nov 23. J Neurophysiol. 2017. PMID: 27881719 Free PMC article.
-
Recurrent Neural Circuits Overcome Partial Inactivation by Compensation and Re-learning.J Neurosci. 2024 Apr 17;44(16):e1635232024. doi: 10.1523/JNEUROSCI.1635-23.2024. J Neurosci. 2024. PMID: 38413233 Free PMC article.
-
Differentiating between integration and non-integration strategies in perceptual decision making.Elife. 2020 Apr 27;9:e55365. doi: 10.7554/eLife.55365. Elife. 2020. PMID: 32338595 Free PMC article.
References
-
- Averbeck B, Latham P, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci 7: 358–366, 2006 - PubMed
-
- Billingsley P. Probability and Measure. Hoboken, NJ: Wiley-Interscience, 1986
-
- Bogacz R, Brown E, Holmes P, Cohen JD. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol Rev 113: 700–765, 2006 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

