The role of chemokines in Guillain-Barré syndrome
- PMID: 23447114
- PMCID: PMC4024180
- DOI: 10.1002/mus.23829
The role of chemokines in Guillain-Barré syndrome
Abstract
Introduction: Chemokines and their receptors are important mediators of inflammation. Guillain-Barré syndrome (GBS) is the most common cause of acute paralysis worldwide. Despite current treatments, outcomes are suboptimal. Specific chemokine receptor antagonists have the potential to be efficacious against pathogenic leukocyte trafficking in GBS.
Methods: A 36-year literature review was performed to summarize available data on chemokine expression in GBS and its representative animal model, experimental autoimmune neuritis (EAN).
Results: Although there were a few observational human and animal studies demonstrating chemokine ligand/receptor expression in GBS and EAN, in vitro and in vivo functional studies using gene knockouts, neutralizing antibodies, or small molecular antagonists were limited. CCL2-CCR2, CCL5-CCR5, and CXCL10-CXCR3 have been most strongly implicated in EAN and GBS pathogenesis, providing targets for molecular blockade.
Conclusions: Preclinical human in vitro and in vivo EAN studies are needed to evaluate the potential efficacy of chemokine signaling inhibition in GBS.
Keywords: Guillain-Barré syndrome; chemokine; chemokine receptor; experimental autoimmune neuritis; therapy.
Copyright © 2013 Wiley Periodicals, Inc.
Figures

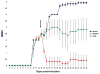
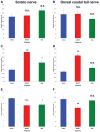
References
-
- Xia RH, Yosef N, Ubogu EE. Selective expression and cellular localization of pro-inflammatory chemokine ligand/receptor pairs in the sciatic nerves of a severe murine experimental autoimmune neuritis model of Guillain-Barré syndrome. Neuropathol Appl Neurobiol. 2010;36:388–398. - PubMed
-
- Brettschneider J, Petzold A, Sussmuth S, Tumani H. Cerebrospinal fluid biomarkers in Guillain-Barré syndrome—where do we stand? J Neurol. 2009;256:3–12. - PubMed
-
- Lu M-O, Zhu J. The role of cytokines in Guillain-Barré syndrome. J Neurol. 2011;258:533–548. - PubMed
-
- Tsang R, Valdivieso-Garcia A. Pathogenesis of Guillain-Barré syndrome. Expert Rev Anti Infect Ther. 2003;1:597–608. - PubMed
-
- van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7:939–950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

