Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors
- PMID: 23447360
- PMCID: PMC3688635
- DOI: 10.1002/ana.23873
Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors
Abstract
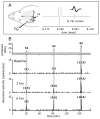
Objective: To examine changes in the response properties of meningeal nociceptors that might lead to migraine pain and examine endogenous processes that could play a role in mediating them using a clinically relevant model of migraine triggering, namely infusion of the nitric oxide (NO) donor nitroglycerin (NTG).
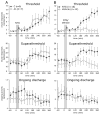
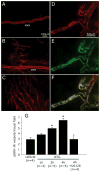
Methods: Single-unit recordings made in the trigeminal ganglion of rats were used to test changes in the activity and mechanosensitivity of meningeal nociceptors in response to administration of the migraine trigger NTG or another NO donor S-nitroso-N-acetyl-DL-penicillamine (SNAP) at doses relevant to the human model of migraine headache. Immunohistochemistry and pharmacological manipulations were used to investigate the possible role of meningeal vascular signaling in mediating the responses of meningeal nociceptors to NO.
Results: Infusion of NTG promoted a delayed and robust increase in the mechanosensitivity of meningeal nociceptors, with a time course resembling the development of the delayed migraine headache. A similar sensitization was elicited by dural application of NTG and SNAP. NTG-evoked delayed meningeal nociceptor sensitization was associated with a robust extracellular signal-regulated kinase (ERK) phosphorylation in meningeal arteries. Pharmacological blockade of meningeal ERK phosphorylation inhibited the development of NTG-evoked delayed meningeal nociceptor sensitization.
Interpretation: The development of delayed mechanical sensitization evoked by the migraine trigger NTG is potentially of great importance as the first finding of a neurophysiological correlate of migraine headache in meningeal nociceptors. The arterial ERK phosphorylation and its involvement in mediating the NTG-evoked delayed sensitization points to an important, yet unappreciated, role of the meningeal vasculature in the genesis of migraine pain.
© 2013 American Neurological Association.
Figures
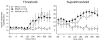
Similar articles
-
Meningeal P2X7 Signaling Mediates Migraine-Related Intracranial Mechanical Hypersensitivity.J Neurosci. 2023 Aug 16;43(33):5975-5985. doi: 10.1523/JNEUROSCI.0368-23.2023. Epub 2023 Jul 24. J Neurosci. 2023. PMID: 37487740 Free PMC article.
-
Tumor necrosis factor-α induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions.Pain. 2011 Jan;152(1):140-149. doi: 10.1016/j.pain.2010.10.002. Epub 2010 Oct 30. Pain. 2011. PMID: 21036476 Free PMC article.
-
Dissociation between CSD-Evoked Metabolic Perturbations and Meningeal Afferent Activation and Sensitization: Implications for Mechanisms of Migraine Headache Onset.J Neurosci. 2018 May 30;38(22):5053-5066. doi: 10.1523/JNEUROSCI.0115-18.2018. Epub 2018 Apr 27. J Neurosci. 2018. PMID: 29703787 Free PMC article.
-
Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: the role of immuno-vascular interactions and cortical spreading depression.Curr Pain Headache Rep. 2012 Jun;16(3):270-7. doi: 10.1007/s11916-012-0255-1. Curr Pain Headache Rep. 2012. PMID: 22328144 Review.
-
Current understanding of meningeal and cerebral vascular function underlying migraine headache.Cephalalgia. 2019 Nov;39(13):1606-1622. doi: 10.1177/0333102418771350. Epub 2018 Jun 21. Cephalalgia. 2019. PMID: 29929378 Review.
Cited by
-
Role of phosphorylated extracellular signal-regulated kinase, calcitonin gene-related peptide and cyclooxygenase-2 in experimental rat models of migraine.Mol Med Rep. 2015 Aug;12(2):1803-9. doi: 10.3892/mmr.2015.3616. Epub 2015 Apr 15. Mol Med Rep. 2015. PMID: 25892078 Free PMC article.
-
Vascular Contributions to Migraine: Time to Revisit?Front Cell Neurosci. 2018 Aug 3;12:233. doi: 10.3389/fncel.2018.00233. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30127722 Free PMC article.
-
MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model.J Neuroinflammation. 2021 Dec 10;18(1):287. doi: 10.1186/s12974-021-02342-5. J Neuroinflammation. 2021. PMID: 34893074 Free PMC article.
-
Unraveling the relationship between inflammation and cluster headache.Front Neurol. 2025 Apr 3;16:1548522. doi: 10.3389/fneur.2025.1548522. eCollection 2025. Front Neurol. 2025. PMID: 40248013 Free PMC article. Review.
-
Sensitization of meningeal afferents to locomotion-related meningeal deformations in a migraine model.Elife. 2024 Feb 8;12:RP91871. doi: 10.7554/eLife.91871. Elife. 2024. PMID: 38329894 Free PMC article.
References
-
- Stovner L, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007 Mar;27(3):193–210. - PubMed
-
- Blau JN, Dexter SL. The site of pain origin during migraine attacks. Cephalalgia. 1981;1(3):143–7. - PubMed
-
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet neurology. 2009 Jul;8(7):679–90. - PubMed
-
- Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. Journal of neurophysiology. 2006 Mar;95(3):1298–306. - PubMed
-
- Messlinger K. Migraine: where and how does the pain originate? Experimental brain research Experimentelle Hirnforschung. 2009 Jun;196(1):179–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

