Mutational tail loss is an evolutionary mechanism for liberating marapsins and other type I serine proteases from transmembrane anchors
- PMID: 23447538
- PMCID: PMC3624440
- DOI: 10.1074/jbc.M112.449033
Mutational tail loss is an evolutionary mechanism for liberating marapsins and other type I serine proteases from transmembrane anchors
Abstract
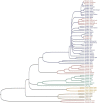
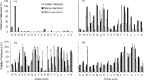
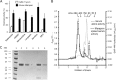
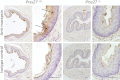
Human and mouse marapsins (Prss27) are serine proteases preferentially expressed by stratified squamous epithelia. However, mouse marapsin contains a transmembrane anchor absent from the human enzyme. To gain insights into physical forms, activities, inhibition, and roles in epithelial differentiation, we traced tail loss in human marapsin to a nonsense mutation in an ancestral ape, compared substrate preferences of mouse and human marapsins with those of the epithelial peptidase prostasin, designed a selective substrate and inhibitor, and generated Prss27-null mice. Phylogenetic analysis predicts that most marapsins are transmembrane proteins. However, nonsense mutations caused membrane anchor loss in three clades: human/bonobo/chimpanzee, guinea pig/degu/tuco-tuco/mole rat, and cattle/yak. Most marapsin-related proteases, including prostasins, are type I transmembrane proteins, but the closest relatives (prosemins) are not. Soluble mouse and human marapsins are tryptic with subsite preferences distinct from those of prostasin, lack general proteinase activity, and unlike prostasins resist antiproteases, including leupeptin, aprotinin, serpins, and α2-macroglobulin, suggesting the presence of non-canonical active sites. Prss27-null mice develop normally in barrier conditions and are fertile without overt epithelial defects, indicating that marapsin does not play critical, non-redundant roles in development, reproduction, or epithelial differentiation. In conclusion, marapsins are conserved, inhibitor-resistant, tryptic peptidases. Although marapsins are type I transmembrane proteins in their typical form, they mutated independently into anchorless forms in several mammalian clades, including one involving humans. Similar pathways appear to have been traversed by prosemins and tryptases, suggesting that mutational tail loss is an important means of evolving new functions of tryptic serine proteases from transmembrane ancestors.
Figures



References
-
- Puente X. S., Sánchez L. M., Overall C. M., López-Otín C. (2003) Human and mouse proteases: a comparative genomic approach. Nat. Rev. Genet. 4, 544–558 - PubMed
-
- Netzel-Arnett S., Hooper J. D., Szabo R., Madison E. L., Quigley J. P., Bugge T. H., Antalis T. M. (2003) Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 22, 237–258 - PubMed
-
- Wong G. W., Stevens R. L. (2005) Identification of a subgroup of glycosylphosphatidylinositol-anchored tryptases. Biochem. Biophys. Res. Commun. 336, 579–584 - PubMed
-
- Wong G. W., Tang Y., Feyfant E., Sali A., Li L., Li Y., Huang C., Friend D. S., Krilis S. A., Stevens R. L. (1999) Identification of a new member of the tryptase family of mouse and human mast cell proteases which possesses a novel COOH-terminal hydrophobic extension. J. Biol. Chem. 274, 30784–30793 - PubMed
-
- Caughey G. H., Raymond W. W., Blount J. L., Hau L. W., Pallaoro M., Wolters P. J., Verghese G. M. (2000) Characterization of human γ-tryptases, novel members of the chromosome 16p mast cell tryptase and prostasin gene families. J. Immunol. 164, 6566–6575 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

