Cytotoxicity of intracellular aβ42 amyloid oligomers involves Ca2+ release from the endoplasmic reticulum by stimulated production of inositol trisphosphate
- PMID: 23447594
- PMCID: PMC3708452
- DOI: 10.1523/JNEUROSCI.4367-12.2013
Cytotoxicity of intracellular aβ42 amyloid oligomers involves Ca2+ release from the endoplasmic reticulum by stimulated production of inositol trisphosphate
Abstract
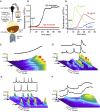
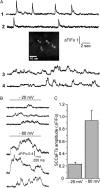
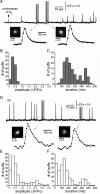
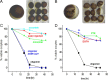
Oligomeric forms of β-amyloid (Aβ(42)) peptides associated with Alzheimer's disease (AD) disrupt cellular Ca(2+) regulation by liberating Ca(2+) into the cytosol from both extracellular and intracellular sources. We elucidated the actions of intracellular Aβ by imaging Ca(2+) responses to injections of Aβ oligomers into Xenopus oocytes. Two types of signal were observed: (1) local, "channel-like" transients dependent on extracellular Ca(2+) influx, which resembled signals from amlyoid pores formed by extracellular application of oligomers; and (2) local transients and global Ca(2+) waves, resembling Ca(2+) puffs and waves mediated by inositol trisphosphate (IP(3)). The latter responses were suppressed by antagonists of the IP(3) receptor (caffeine and heparin), pretreatment with the G(i)(o)-protein inhibitor pertussis toxin, and pretreatment with lithium to deplete membrane inositol lipids. We show that G-protein-mediated stimulation of IP(3) production and consequent liberation of Ca(2+) from the endoplasmic reticulum by intracellular Aβ oligomers is cytotoxic, potentially representing a novel pathological mechanism in AD which may be further exacerbated by AD-linked mutations in presenilins to promote opening of IP(3) receptor/channels.
Figures



References
-
- Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, Domercq M, Matute C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–272. - PubMed
-
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
-
- Berridge MJ. Calcium hypothesis of Alzheimer's disease. Pflugers Arch. 2010;459:441–449. - PubMed
-
- Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
