Identification of nonvisual photomotor response cells in the vertebrate hindbrain
- PMID: 23447595
- PMCID: PMC3600642
- DOI: 10.1523/JNEUROSCI.3689-12.2013
Identification of nonvisual photomotor response cells in the vertebrate hindbrain
Abstract
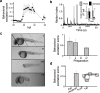
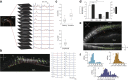
Nonvisual photosensation enables animals to sense light without sight. However, the cellular and molecular mechanisms of nonvisual photobehaviors are poorly understood, especially in vertebrate animals. Here, we describe the photomotor response (PMR), a robust and reproducible series of motor behaviors in zebrafish that is elicited by visual wavelengths of light but does not require the eyes, pineal gland, or other canonical deep-brain photoreceptive organs. Unlike the relatively slow effects of canonical nonvisual pathways, motor circuits are strongly and quickly (seconds) recruited during the PMR behavior. We find that the hindbrain is both necessary and sufficient to drive these behaviors. Using in vivo calcium imaging, we identify a discrete set of neurons within the hindbrain whose responses to light mirror the PMR behavior. Pharmacological inhibition of the visual cycle blocks PMR behaviors, suggesting that opsin-based photoreceptors control this behavior. These data represent the first known light-sensing circuit in the vertebrate hindbrain.
Figures




References
-
- Becker HE, Cone RA. Light-stimulated electrical responses from skin. Science. 1966;154:1051–1053. - PubMed
-
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. - PubMed
-
- Best JD, Berghmans S, Hunt JJ, Clarke SC, Fleming A, Goldsmith P, Roach AG. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33:1206–1215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 MH085205/MH/NIMH NIH HHS/United States
- DP1 NS082121/NS/NINDS NIH HHS/United States
- R01MH086867/MH/NIMH NIH HHS/United States
- K01MH091449/MH/NIMH NIH HHS/United States
- R01 CA140188/CA/NCI NIH HHS/United States
- K01 MH091449/MH/NIMH NIH HHS/United States
- R01 DA030304/DA/NIDA NIH HHS/United States
- DP1 NS082121/DP/NCCDPHP CDC HHS/United States
- T32 MH020017/MH/NIMH NIH HHS/United States
- R01 MH086867/MH/NIMH NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R21MH085205/MH/NIMH NIH HHS/United States
- R01DA030304/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
