Simultaneous top-down modulation of the primary somatosensory cortex and thalamic nuclei during active tactile discrimination
- PMID: 23447616
- PMCID: PMC3674835
- DOI: 10.1523/JNEUROSCI.1659-12.2013
Simultaneous top-down modulation of the primary somatosensory cortex and thalamic nuclei during active tactile discrimination
Abstract
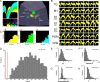
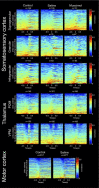
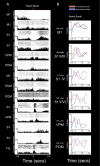
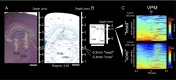
The rat somatosensory system contains multiple thalamocortical loops (TCLs) that altogether process, in fundamentally different ways, tactile stimuli delivered passively or actively sampled. To elucidate potential top-down mechanisms that govern TCL processing in awake, behaving animals, we simultaneously recorded neuronal ensemble activity across multiple cortical and thalamic areas while rats performed an active aperture discrimination task. Single neurons located in the primary somatosensory cortex (S1), the ventroposterior medial, and the posterior medial thalamic nuclei of the trigeminal somatosensory pathways exhibited prominent anticipatory firing modulations before the whiskers touching the aperture edges. This cortical and thalamic anticipatory firing could not be explained by whisker movements or whisker stimulation, because neither trigeminal ganglion sensory-evoked responses nor EMG activity were detected during the same period. Both thalamic and S1 anticipatory activity were predictive of the animal's discrimination accuracy. Inactivation of the primary motor cortex (M1) with muscimol affected anticipatory patterns in S1 and the thalamus, and impaired the ability to predict the animal's performance accuracy based on thalamocortical anticipatory activity. These findings suggest that neural processing in TCLs is launched in anticipation of whisker contact with objects, depends on top-down effects generated in part by M1 activity, and cannot be explained by the classical feedforward model of the rat trigeminal system.
Figures

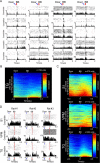
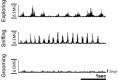
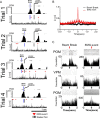
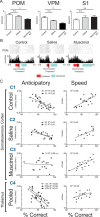
Comment in
-
Brain dynamical networks: contextualizing the function of primary cortices.J Neurosci. 2013 Jun 26;33(26):10588-90. doi: 10.1523/JNEUROSCI.1768-13.2013. J Neurosci. 2013. PMID: 23804082 Free PMC article. No abstract available.
References
-
- Arkley K, Grant R, Mitchinson B, Prescott T. The relationship between whisking and locomotion in rats. Soc Neurosci Abstr. 2011;37:76.24.
-
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol. 1987;263:265–281. - PubMed
-
- Belford GR, Killackey HP. Vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979;183:305–321. - PubMed
-
- Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004;427:704–710. - PubMed
-
- Chapin JK, Woodward DJ. Modulation of sensory responsiveness of single somatosensory cortical cells during movement and arousal behaviors. Exp Neurol. 1981;72:164–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
