Mechanistic and structural determinants of NMDA receptor voltage-dependent gating and slow Mg2+ unblock
- PMID: 23447622
- PMCID: PMC3629906
- DOI: 10.1523/JNEUROSCI.3712-12.2013
Mechanistic and structural determinants of NMDA receptor voltage-dependent gating and slow Mg2+ unblock
Abstract
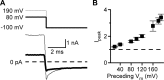
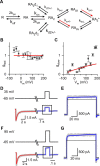
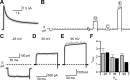
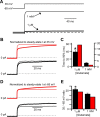
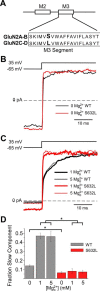
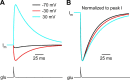
NMDA receptor (NMDAR)-mediated currents depend on membrane depolarization to relieve powerful voltage-dependent NMDAR channel block by external magnesium (Mg(o)(2+)). Mg(o)(2+) unblock from native NMDARs exhibits a fast component that is consistent with rapid Mg(o)(2+) -unbinding kinetics and also a slower, millisecond time scale component (slow Mg(o)(2+) unblock). In recombinant NMDARs, slow Mg(o)(2+) unblock is prominent in GluN1/2A (an NMDAR subtype composed of GluN1 and GluN2A subunits) and GluN1/2B receptors, with slower kinetics observed for GluN1/2B receptors, but absent from GluN1/2C and GluN1/2D receptors. Slow Mg(o)(2+) unblock from GluN1/2B receptors results from inherent voltage-dependent gating, which increases channel open probability with depolarization. Here we examine the mechanisms responsible for NMDAR subtype dependence of slow Mg(o)(2+) unblock. We demonstrate that slow Mg(o)(2+) unblock from GluN1/2A receptors, like GluN1/2B receptors, results from inherent voltage-dependent gating. Surprisingly, GluN1/2A and GluN1/2B receptors exhibited equal inherent voltage dependence; faster Mg(o)(2+) unblock from GluN1/2A receptors can be explained by voltage-independent differences in gating kinetics. To investigate the absence of slow Mg(o)(2+) unblock in GluN1/2C and GluN1/2D receptors, we examined the GluN2 S/L site, a site responsible for several NMDAR subtype-dependent channel properties. Mutating the GluN2 S/L site of GluN2A subunits from serine (found in GluN2A and GluN2B subunits) to leucine (found in GluN2C and GluN2D) greatly diminished both voltage-dependent gating and slow Mg(o)(2+) unblock. Therefore, the residue at the GluN2 S/L site governs the expression of both slow Mg(o)(2+) unblock and inherent voltage dependence.
Figures

References
-
- Antonov SM, Gmiro VE, Johnson JW. Binding sites for permeant ions in the channel of NMDA receptors and their effects on channel block. Nat Neurosci. 1998;1:451–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
