Tbr2 expression in Cajal-Retzius cells and intermediate neuronal progenitors is required for morphogenesis of the dentate gyrus
- PMID: 23447624
- PMCID: PMC3623668
- DOI: 10.1523/JNEUROSCI.4185-12.2013
Tbr2 expression in Cajal-Retzius cells and intermediate neuronal progenitors is required for morphogenesis of the dentate gyrus
Abstract
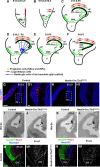
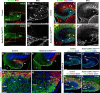
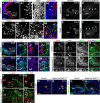
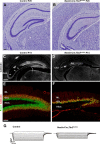
The dentate gyrus (DG) is a unique cortical region whose protracted development spans the embryonic and early postnatal periods. DG development involves large-scale reorganization of progenitor cell populations, ultimately leading to the establishment of the subgranular zone neurogenic niche. In the developing DG, the T-box transcription factor Tbr2 is expressed in both Cajal-Retzius cells derived from the cortical hem that guide migration of progenitors and neurons to the DG, and intermediate neuronal progenitors born in the dentate neuroepithelium that give rise to granule neurons. Here we show that in mice Tbr2 is required for proper migration of Cajal-Retzius cells to the DG; and, in the absence of Tbr2, formation of the hippocampal fissure is abnormal, leading to aberrant development of the transhilar radial glial scaffold and impaired migration of progenitors and neuroblasts to the developing DG. Furthermore, loss of Tbr2 results in decreased expression of Cxcr4 in migrating cells, leading to a premature burst of granule neurogenesis during early embryonic development accompanied by increased cell death in mutant animals. Formation of the transient subpial neurogenic zone was abnormal in Tbr2 conditional knock-outs, and the stem cell population in the DG was depleted before proper establishment of the subgranular zone. These studies indicate that Tbr2 is explicitly required for morphogenesis of the DG and participates in multiple aspects of the intricate developmental process of this structure.
Figures




Similar articles
-
Bone morphogenic protein signaling is a major determinant of dentate development.J Neurosci. 2013 Apr 17;33(16):6766-75. doi: 10.1523/JNEUROSCI.0128-13.2013. J Neurosci. 2013. PMID: 23595735 Free PMC article.
-
DNA Methyltransferase 1 Is Indispensable for Development of the Hippocampal Dentate Gyrus.J Neurosci. 2016 Jun 1;36(22):6050-68. doi: 10.1523/JNEUROSCI.0512-16.2016. J Neurosci. 2016. PMID: 27251626 Free PMC article.
-
CXCR4 prevents dispersion of granule neuron precursors in the adult dentate gyrus.Hippocampus. 2013 Dec;23(12):1345-58. doi: 10.1002/hipo.22180. Epub 2013 Sep 10. Hippocampus. 2013. PMID: 23929505
-
Epigenetic regulation in the neurogenic niche of the adult dentate gyrus.Neurosci Lett. 2022 Jan 1;766:136343. doi: 10.1016/j.neulet.2021.136343. Epub 2021 Nov 11. Neurosci Lett. 2022. PMID: 34774980 Free PMC article. Review.
-
Radial glia, the keystone of the development of the hippocampal dentate gyrus.Mol Neurobiol. 2015 Feb;51(1):131-41. doi: 10.1007/s12035-014-8692-y. Epub 2014 Apr 10. Mol Neurobiol. 2015. PMID: 24719081 Review.
Cited by
-
Evolution of the mammalian dentate gyrus.J Comp Neurol. 2016 Feb 15;524(3):578-94. doi: 10.1002/cne.23851. Epub 2015 Jul 29. J Comp Neurol. 2016. PMID: 26179319 Free PMC article. Review.
-
Cell-Biological Requirements for the Generation of Dentate Gyrus Granule Neurons.Front Cell Neurosci. 2018 Nov 12;12:402. doi: 10.3389/fncel.2018.00402. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30483057 Free PMC article. Review.
-
Cajal-Retzius neurons are required for the development of the human hippocampal fissure.J Anat. 2019 Sep;235(3):569-589. doi: 10.1111/joa.12947. Epub 2019 Mar 12. J Anat. 2019. PMID: 30861578 Free PMC article.
-
Spatially resolved cell atlas of the teleost telencephalon and deep homology of the vertebrate forebrain.bioRxiv [Preprint]. 2023 Jul 22:2023.07.20.549873. doi: 10.1101/2023.07.20.549873. bioRxiv. 2023. Update in: Commun Biol. 2024 May 21;7(1):612. doi: 10.1038/s42003-024-06315-1. PMID: 37503039 Free PMC article. Updated. Preprint.
-
Deconstructing Sox2 Function in Brain Development and Disease.Cells. 2022 May 10;11(10):1604. doi: 10.3390/cells11101604. Cells. 2022. PMID: 35626641 Free PMC article. Review.
References
-
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. - PubMed
-
- Berger O, Li G, Han SM, Paredes M, Pleasure SJ. Expression of SDF-1 and CXCR4 during reorganization of the postnatal dentate gyrus. Dev Neurosci. 2007;29:48–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
