Mitochondrial aldehyde dehydrogenase-2 activation prevents β-amyloid-induced endothelial cell dysfunction and restores angiogenesis
- PMID: 23447675
- PMCID: PMC3666252
- DOI: 10.1242/jcs.117184
Mitochondrial aldehyde dehydrogenase-2 activation prevents β-amyloid-induced endothelial cell dysfunction and restores angiogenesis
Abstract
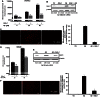
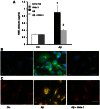
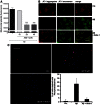
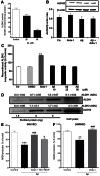
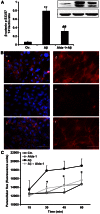
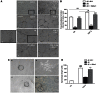
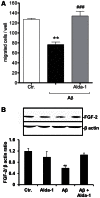
Amyloid β peptides (Aβ1-40 and Aβ1-42) cause cerebral degeneration by impairing the activity of angiogenic factors and inducing apoptosis and senescence in the endothelium. Amyloid peptides are known to induce oxidative stress. Impairment of mitochondrial aldehyde dehydrogenase 2 (ALDH2) following oxidative stress, results in accumulation of toxic aldehydes, particularly 4-hydroxynoneal (4-HNE). We sought to determine the role of mitochondrial ALDH2 in Aβ-related impairment of angiogenesis. We hypothesized that by increasing the detoxification activity of ALDH2 we would reduce Aβ-driven endothelial injuries and restore angiogenesis. We used a selective ALDH2 activator, Alda-1, assessing its ability to repair mitochondrial dysfunction in the endothelium. Treatment of human endothelial cells with Aβ1-40 (5-50 µM) induced loss of mitochondrial membrane potential, increased cytochrome c release and ROS accumulation. These events were associated with 4-HNE accumulation and decrease in ALDH2 activity (40%), and resulted in disassembly of endothelial junctions, as evidenced by β-catenin phosphorylation, disorganization of adherens and tight junctions, and by disruption of pseudocapillary formation. Alda-1 (10-40 µM) abolished Aβ-induced 4-HNE accumulation, apoptosis and vascular leakiness, fully restoring the pro-angiogenic endothelial phenotype and responses to FGF-2. Our data document that mitochondrial ALDH2 in the endothelium is a target for the vascular effect of Aβ, including loss of barrier function and angiogenesis. ALDH2 activation, by restoring mitochondrial functions in the endothelium, prevents Aβ-induced dysfunction and anti-angiogenic effects. Thus, agents activating ALDH2 may reduce endothelial injuries including those occurring in cerebral amyloid angiopathy, preserving the angiogenic potential of the endothelium.
Keywords: Aldehyde dehydrogenase 2; Amyloid β; Angiogenesis; Mitochondria.
Figures
References
-
- Aliev G., Seyidova D., Lamb B. T., Obrenovich M. E., Siedlak S. L., Vinters H. V., Friedland R. P., LaManna J. C., Smith M. A., Perry G. (2003). Mitochondria and vascular lesions as a central target for the development of Alzheimer's disease and Alzheimer disease-like pathology in transgenic mice. Neurol. Res. 25, 665–674 10.1179/016164103101201977 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

