The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes
- PMID: 23447677
- PMCID: PMC3666251
- DOI: 10.1242/jcs.113225
The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes
Abstract
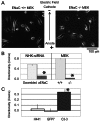
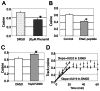
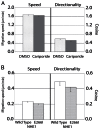
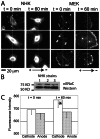
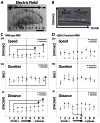
Cellular directional migration in an electric field (galvanotaxis) is one of the mechanisms guiding cell movement in embryogenesis and in skin epidermal repair. The epithelial sodium channel (ENaC), in addition to its function of regulating sodium transport in kidney, has recently been found to modulate cell locomotory speed. Here we tested whether ENaC has an additional function of mediating the directional migration of galvanotaxis in keratinocytes. Genetic depletion of ENaC completely blocks only galvanotaxis and does not decrease migration speed. Overexpression of ENaC is sufficient to drive galvanotaxis in otherwise unresponsive cells. Pharmacologic blockade or maintenance of the open state of ENaC also decreases or increases, respectively, galvanotaxis, suggesting that the channel open state is responsible for the response. Stable lamellipodial extensions formed at the cathodal sides of wild-type cells at the start of galvanotaxis; these were absent in the ENaC knockout keratinocytes, suggesting that ENaC mediates galvanotaxis by generating stable lamellipodia that steer cell migration. We provide evidence that ENaC is required for directional migration of keratinocytes in an electric field, supporting a role for ENaC in skin wound healing.
Keywords: Directional migration; ENaC; Keratinocyte; Lamellipodia.
Figures
Similar articles
-
Calcium channel blockers inhibit galvanotaxis in human keratinocytes.J Cell Physiol. 2002 Oct;193(1):1-9. doi: 10.1002/jcp.10144. J Cell Physiol. 2002. PMID: 12209874
-
Cyclic AMP mediates keratinocyte directional migration in an electric field.J Cell Sci. 2005 May 1;118(Pt 9):2023-34. doi: 10.1242/jcs.02330. Epub 2005 Apr 19. J Cell Sci. 2005. PMID: 15840650
-
Hydration status regulates sodium flux and inflammatory pathways through epithelial sodium channel (ENaC) in the skin.J Invest Dermatol. 2015 Mar;135(3):796-806. doi: 10.1038/jid.2014.477. Epub 2014 Nov 5. J Invest Dermatol. 2015. PMID: 25371970
-
Regulation of the epithelial Na+ channel by peptidases.Curr Top Dev Biol. 2007;78:23-46. doi: 10.1016/S0070-2153(06)78002-4. Curr Top Dev Biol. 2007. PMID: 17338914 Free PMC article. Review.
-
Regulation of epithelial sodium transport via epithelial Na+ channel.J Biomed Biotechnol. 2011;2011:978196. doi: 10.1155/2011/978196. Epub 2011 Oct 17. J Biomed Biotechnol. 2011. PMID: 22028593 Free PMC article. Review.
Cited by
-
The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na(+) channel (ENaC): IUPHAR Review 19.Br J Pharmacol. 2016 Sep;173(18):2671-701. doi: 10.1111/bph.13533. Epub 2016 Aug 10. Br J Pharmacol. 2016. PMID: 27278329 Free PMC article. Review.
-
Electrotaxis evokes directional separation of co-cultured keratinocytes and fibroblasts.Sci Rep. 2023 Jul 15;13(1):11444. doi: 10.1038/s41598-023-38664-y. Sci Rep. 2023. PMID: 37454232 Free PMC article.
-
Effects of direct current electric-field using ITO plate on breast cancer cell migration.Biomater Res. 2014 Jul 31;18:10. doi: 10.1186/2055-7124-18-10. eCollection 2014. Biomater Res. 2014. PMID: 26331061 Free PMC article.
-
Electroceutical Management of Bacterial Biofilms and Surgical Infection.Antioxid Redox Signal. 2020 Oct 1;33(10):713-724. doi: 10.1089/ars.2020.8086. Epub 2020 Jul 10. Antioxid Redox Signal. 2020. PMID: 32466673 Free PMC article.
-
Alpha and beta adrenergic receptors modulate keratinocyte migration.PLoS One. 2021 Jul 2;16(7):e0253139. doi: 10.1371/journal.pone.0253139. eCollection 2021. PLoS One. 2021. PMID: 34214097 Free PMC article.
References
-
- Awayda M. S., Ismailov I. I., Berdiev B. K., Benos D. J. (1995). A cloned renal epithelial Na+ channel protein displays stretch activation in planar lipid bilayers. Am. J. Physiol. 268, C1450–C1459 - PubMed
-
- Awayda M. S., Tousson A., Benos D. J. (1997). Regulation of a cloned epithelial Na+ channel by its beta- and gamma-subunits. Am. J. Physiol. 273, C1889–C1899 - PubMed
-
- Barker A. T., Jaffe L. F., Vanable J. W., Jr (1982). The glabrous epidermis of cavies contains a powerful battery. Am. J. Physiol. 242, R358–R366 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

