Cutting edge: Leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus
- PMID: 23447682
- PMCID: PMC3608794
- DOI: 10.4049/jimmunol.1203275
Cutting edge: Leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus
Abstract
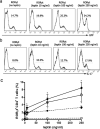
Th17 CD4(+) cells promote inflammation and autoimmunity. In this study, we report that Th17 cell frequency is reduced in ob/ob mice (that are genetically deficient in the adipokine leptin) and that the administration of leptin to ob/ob mice restored Th17 cell numbers to values comparable to those found in wild-type animals. Leptin promoted Th17 responses in normal human CD4(+) T cells and in mice, both in vitro and in vivo, by inducing RORγt transcription. Leptin also increased Th17 responses in (NZB × NZW)F1 lupus-prone mice, whereas its neutralization in those autoimmune-prone mice inhibited Th17 responses. Because Th17 cells play an important role in the development and maintenance of inflammation and autoimmunity, these findings envision the possibility to modulate abnormal Th17 responses via leptin manipulation, and they reiterate the link between metabolism/nutrition and susceptibility to autoimmunity.
Figures

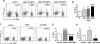
References
-
- Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW. Crystal structure of the obese protein leptin-E100. Nature. 1997;387:206–209. - PubMed
-
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. - PubMed
-
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. - PubMed
-
- La Cava A, Matarese G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004;4:371–379. - PubMed
-
- De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

