Class I phosphoinositide-3-kinases and SRC kinases play a nonredundant role in regulation of adhesion-independent and -dependent neutrophil reactive oxygen species generation
- PMID: 23447687
- PMCID: PMC4280093
- DOI: 10.4049/jimmunol.1201951
Class I phosphoinositide-3-kinases and SRC kinases play a nonredundant role in regulation of adhesion-independent and -dependent neutrophil reactive oxygen species generation
Abstract
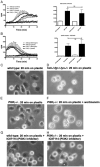
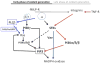
Chemoattractant-induced reactive oxygen species (ROS) generation by adherent neutrophils occurs in two phases: the first is very rapid and transient, and the second one is delayed and lasts up to 30-40 min. We examined the role of phosphoinositide 3-kinases (PI3Ks) and Src-family kinases (SFKs) in these responses using human neutrophils treated with inhibitory compounds or murine neutrophils deficient of PI3Kγ or Hck, Fgr, and Lyn. Our studies show that PI3Kγ is indispensable for the early, fMLF-induced ROS generation and AKT and ERK phosphorylation, but is dispensable for the late response to fMLF. Additionally, the response to TNF, an agonist triggering only the delayed phase of ROS generation, was also unaffected in PI3Kγ-deficient neutrophils. In contrast, inhibition of SFKs by a selective inhibitor in human, or SFK deficiency in murine, neutrophils resulted in the inhibition of both the early and late phase of ROS generation, without affecting the early phase of AKT phosphorylation, but inhibiting the late one. Selective inhibitors of PI3Kα and PI3Kδ markedly reduced both the early and late response to fMLF and TNF in human neutrophils. These findings suggest that class IA PI3Ks may be activated by PI3Kγ via Ras in the early phase of the response and by SFKs in the late phase. The evidence that inhibition of SFKs in human, or SFK deficiency in murine, neutrophils results in suppression of Vav phosphorylation at all time points of the response to fMLF or TNF suggests that SFKs are indispensable for Vav phosphorylation.
Figures
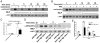

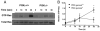



References
-
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. - PubMed
-
- Nathan C, Ding A. SnapShot: Reactive Oxygen Intermediates (ROI) Cell. 2010;140:951–951.e2. - PubMed
-
- Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cell Signal. 1999;11:621–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

