Tetraiodothyroacetic acid-conjugated PLGA nanoparticles: a nanomedicine approach to treat drug-resistant breast cancer
- PMID: 23448245
- PMCID: PMC3825799
- DOI: 10.2217/nnm.12.200
Tetraiodothyroacetic acid-conjugated PLGA nanoparticles: a nanomedicine approach to treat drug-resistant breast cancer
Retraction in
-
Statement of Retraction: Tetraiodothyroacetic acid-conjugated PLGA nanoparticles: a nanomedicine approach to treat drug-resistant breast cancer.Nanomedicine (Lond). 2025 May;20(9):1071. doi: 10.1080/17435889.2025.2488633. Epub 2025 Apr 3. Nanomedicine (Lond). 2025. PMID: 40178493 Free PMC article. No abstract available.
Abstract
Aim: The aim was to evaluate tetraiodothyroacetic acid (tetrac), a thyroid hormone analog of L-thyroxin, conjugated to poly(lactic-co-glycolic acid) nanoparticles (T-PLGA-NPs) both in vitro and in vivo for the treatment of drug-resistant breast cancer.
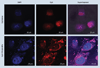
Materials & methods: The uptake of tetrac and T-PLGA-NPs in doxorubicin-resistant MCF7 (MCF7-Dx) cells was evaluated using confocal microscopy. Cell proliferation assays and a chick chorioallantoic membrane model of FGF2-induced angiogenesis were used to evaluate the anticancer effects of T-PLGA-NPs. In vivo efficacy was examined in a MCF7-Dx orthotopic tumor BALBc nude mouse model.
Results: T-PLGA-NPs were restricted from entering into the cell nucleus, and T-PLGA-NPs inhibited angiogenesis by 100% compared with 60% by free tetrac. T-PLGA-NPs enhanced inhibition of tumor-cell proliferation at a low-dose equivalent of free tetrac. In vivo treatment with either tetrac or T-PLGA-NPs resulted in a three- to five-fold inhibition of tumor weight.
Conclusion: T-PLGA-NPs have high potential as anticancer agents, with possible applications in the treatment of drug-resistant cancer.
Figures




Comment in
-
Findings of Research Misconduct.Fed Regist. 2024 Jun 4;89(108):47968-47969. Fed Regist. 2024. PMID: 38854442 Free PMC article. No abstract available.
References
-
- Bharali DJ, Mousa SA. Emerging nanomedicines for early cancer detection, improved treatment: current perspective future promise. Pharmacol. Ther. 2010;128(2):324–335. - PubMed
-
- Brannon-Peppas L, Blanchette JO. Nanoparticle targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004;56(11):1649–1659. - PubMed
-
- Kim K, Kim JH, Park H, et al. Tumor-homing multifunctional nanoparticles for cancer theragnosis: simultaneous diagnosis drug delivery therapeutic monitoring. J. Control. Release. 2010;146(2):219–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
