Safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2239633, a CC-chemokine receptor 4 antagonist, in healthy male subjects: results from an open-label and from a randomised study
- PMID: 23448278
- PMCID: PMC3599276
- DOI: 10.1186/2050-6511-14-14
Safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2239633, a CC-chemokine receptor 4 antagonist, in healthy male subjects: results from an open-label and from a randomised study
Abstract
Background: The CC-chemokine receptor 4 (CCR4) is thought potentially to play a critical role in asthma pathogenesis due to its ability to recruit type 2 T-helper lymphocytes to the inflamed airways. Therefore, CCR4 provides an excellent target for anti-inflammatory therapy.
Methods: The safety, tolerability, pharmacokinetics and pharmacodynamics of the CCR4 antagonist GSK2239633, N-(3-((3-(5-chlorothiophene-2-sulfonamido)-4-methoxy-1H-indazol-1-yl)methyl)benzyl)-2-hydroxy-2-methylpropanamide, were examined in healthy males. Two studies were performed: 1) an open-label, study in which six subjects received a single intravenous infusion of [14C]-GSK2239633 100 μg (10 kBq) (NCT01086462), and 2) a randomised, double-blind, placebo-controlled, cross-over, ascending dose study in which 24 subjects received single oral doses of GSK2239633 150-1500 mg (NCT01371812).
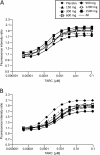
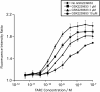
Results: Following intravenous dosing, plasma GSK2239633 displayed rapid, bi-phasic distribution and slow terminal elimination (t½: 13.5 hours), suggesting that GSK2239633 was a low to moderate clearance drug. Following oral dosing, blood levels of GSK2239633 reached Cmax rapidly (median tmax: 1.0-1.5 hours). Estimated GSK2239633 bioavailability was low with a maximum value determined of only 16%. Food increased GSK2239633 systemic exposure (as assessed by AUC and Cmax). Increases in AUC and Cmax were less than dose proportional. Adverse events were reported by three subjects (50%) following intravenous administration, and by 19 subjects (79%) following oral administration; most (46/47; 98%) events were mild/moderate in intensity. GSK2239633 1500 mg inhibited thymus- and activation-regulated chemokine-induced (TARC) actin polymerisation reaching a mean CCR4 occupancy of 74%.
Conclusion: In conclusion, GSK2239633 was well-tolerated and capable of inhibiting TARC from activating the CCR4 receptor.
Figures
References
-
- Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, Assoufi B, Collins JV, Durham S, Kay AB. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142:1407–1413. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

