Hydrosilylated porous silicon particles function as an intravitreal drug delivery system for daunorubicin
- PMID: 23448595
- PMCID: PMC3669596
- DOI: 10.1089/jop.2012.0205
Hydrosilylated porous silicon particles function as an intravitreal drug delivery system for daunorubicin
Abstract
Purpose: To evaluate in vivo ocular safety of an intravitreal hydrosilylated porous silicon (pSi) drug delivery system along with the payload of daunorubicin (DNR).
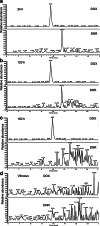
Methods: pSi microparticles were prepared from the electrochemical etching of highly doped, p-type Si wafers and an organic linker was attached to the Si-H terminated inner surface of the particles by thermal hydrosilylation of undecylenic acid. DNR was bound to the carboxy terminus of the linker as a drug-loading strategy. DNR release from hydrosilylated pSi particles was confirmed in the excised rabbit vitreous using liquid chromatography-electrospray ionization-multistage mass spectrometry. Both empty and DNR-loaded hydrosilylated pSi particles were injected into the rabbit vitreous and the degradation and safety were studied for 6 months.
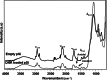
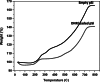
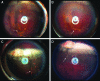
Results: The mean pSi particle size was 30×46×15 μm with an average pore size of 15 nm. Drug loading was determined as 22 μg per 1 mg of pSi particles. An ex vivo drug release study showed that intact DNR was detected in the rabbit vitreous. An in vivo ocular toxicity study did not reveal clinical or pathological evidence of any toxicity during a 6-month observation. Hydrosilylated pSi particles, either empty or loaded with DNR, demonstrated a slow elimination kinetics from the rabbit vitreous without ocular toxicity.
Conclusions: Hydrosilylated pSi particles can host a large quantity of DNR by a covalent loading strategy and DNR can be slowly released into the vitreous without ocular toxicity, which would appear if an equivalent quantity of free drug was injected.
Figures


Similar articles
-
Tunable sustained intravitreal drug delivery system for daunorubicin using oxidized porous silicon.J Control Release. 2014 Mar 28;178:46-54. doi: 10.1016/j.jconrel.2014.01.003. Epub 2014 Jan 11. J Control Release. 2014. PMID: 24424270 Free PMC article.
-
A Novel Approach of Daunorubicin Application on Formation of Proliferative Retinopathy Using a Porous Silicon Controlled Delivery System: Pharmacodynamics.Invest Ophthalmol Vis Sci. 2015 Apr;56(4):2755-63. doi: 10.1167/iovs.15-16526. Invest Ophthalmol Vis Sci. 2015. PMID: 25829415 Free PMC article.
-
Oxidized porous silicon particles covalently grafted with daunorubicin as a sustained intraocular drug delivery system.Invest Ophthalmol Vis Sci. 2013 Feb 1;54(2):1268-79. doi: 10.1167/iovs.12-11172. Invest Ophthalmol Vis Sci. 2013. PMID: 23322571 Free PMC article.
-
Surface chemistry of porous silicon and implications for drug encapsulation and delivery applications.Adv Colloid Interface Sci. 2012 Jul 15;175:25-38. doi: 10.1016/j.cis.2012.03.006. Epub 2012 Mar 30. Adv Colloid Interface Sci. 2012. PMID: 22521238 Review.
-
Recent advances in porous silicon technology for drug delivery.Ther Deliv. 2013 Jul;4(7):811-23. doi: 10.4155/tde.13.52. Ther Deliv. 2013. PMID: 23883125 Review.
Cited by
-
A sustained intravitreal drug delivery system with remote real time monitoring capability.Acta Biomater. 2015 Sep;24:309-21. doi: 10.1016/j.actbio.2015.06.012. Epub 2015 Jun 15. Acta Biomater. 2015. PMID: 26087110 Free PMC article.
-
Tunable sustained intravitreal drug delivery system for daunorubicin using oxidized porous silicon.J Control Release. 2014 Mar 28;178:46-54. doi: 10.1016/j.jconrel.2014.01.003. Epub 2014 Jan 11. J Control Release. 2014. PMID: 24424270 Free PMC article.
-
Effective treatment of retinal neovascular leakage with fusogenic porous silicon nanoparticles delivering VEGF-siRNA.Nanomedicine (Lond). 2022 Nov;17(27):2089-2108. doi: 10.2217/nnm-2022-0255. Epub 2023 Feb 7. Nanomedicine (Lond). 2022. PMID: 36748946 Free PMC article.
-
Mechanism of erosion of nanostructured porous silicon drug carriers in neoplastic tissues.Nat Commun. 2015 Feb 11;6:6208. doi: 10.1038/ncomms7208. Nat Commun. 2015. PMID: 25670235 Free PMC article.
-
Sustained delivery of a HIF-1 antagonist for ocular neovascularization.J Control Release. 2013 Dec 28;172(3):625-33. doi: 10.1016/j.jconrel.2013.10.008. Epub 2013 Oct 12. J Control Release. 2013. PMID: 24126220 Free PMC article.
References
-
- Chappelow A.V. Kaiser P.K. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68:1029–1036. - PubMed
-
- Taban M. Lowder C.Y. Kaiser P.K. Outcome of fluocinolone acetonide implant (retisert(tm)) reimplantation for chronic noninfectious posterior uveitis. Retina. 2008;28:1280–1288. - PubMed
-
- Lee S.S. Robinson M.R. Novel drug delivery systems for retinal diseases. Ophthalmic Res. 2009;41:124–135. - PubMed
-
- MacCumber M.W. Sadeghi S. Cohen J.A. Deutsch T.A. Suture loop to aid in ganciclovir implant removal. Arch. Ophthalmol. 1999;117:1250–1254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

