Molecular immunologic correlates of spontaneous latency in a rabbit model of pulmonary tuberculosis
- PMID: 23448601
- PMCID: PMC3598925
- DOI: 10.1186/1478-811X-11-16
Molecular immunologic correlates of spontaneous latency in a rabbit model of pulmonary tuberculosis
Abstract
Background: Infection of humans with Mycobacterium tuberculosis (Mtb) results in latent tuberculosis infection (LTBI) in 90-95% of immune competent individuals, with no symptoms of active disease. The World Health Organization estimates that 1.5 billion people have LTBI, which can reactivate in the setting of waning host immunity, posing a threat to global TB control. Various animal models have been used to study the pathogenesis of TB. However, besides nonhuman primates, rabbits are the only animal model that fully recapitulates the pathological features of human TB, including progressive disease with necrosis and cavitation or establishment of spontaneous latency.
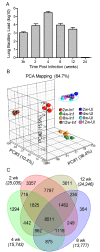
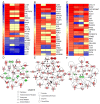
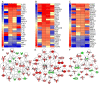
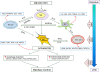
Results: We defined the molecular immunological correlates of LTBI establishment in a rabbit model of pulmonary infection with Mtb CDC1551. After aerosol infection, exponential bacterial growth was noted in the lungs for 4 weeks, followed by a significant decline by 12 weeks, resulting in the absence of cultivable bacilli by 24 weeks. We used rabbit whole genome microarrays to profile the lung transcriptome during the course of infection. At 2 weeks post-infection, gene networks involved in natural killer (NK) and dendritic cell (DC) activation and macrophage antimicrobial activities were highly upregulated. This was followed by upregulation of gene networks involved in macrophage and T cell activation and autophagy, peaking at 4 to 8 weeks. Concomitantly, host Th1, but not Th2 or inflammatory, immune response genes were significantly upregulated. Thus, the expression kinetics of genes involved in cross-talk between innate and adaptive immunity over the first 8 weeks post-infection were consistent with early efficient control of infection in the lungs. Interestingly, expression of many genes of the host innate and adaptive immune response pathways was downregulated at 12 weeks, suggesting that immune activation did not persist once bacilli began to clear from the infected lungs.
Conclusions: Our results suggest that early activation of host innate immunity prior to efficient activation of T cell-mediated adaptive immunity but not inflammation is essential for establishment of LTBI in Mtb CDC1551-infected rabbits. We also show that T cell activation and the host adaptive immune response networks are dampened once bacterial growth is controlled, ultimately resulting in spontaneous LTBI.
Figures

References
-
- WHO. Global tuberculosis control: WHO report 2011. Geneva, Switzerland: World Health Organization; 2011.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

