Neuroblastoma tumorigenesis is regulated through the Nm23-H1/h-Prune C-terminal interaction
- PMID: 23448979
- PMCID: PMC3584926
- DOI: 10.1038/srep01351
Neuroblastoma tumorigenesis is regulated through the Nm23-H1/h-Prune C-terminal interaction
Abstract
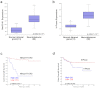
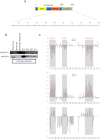
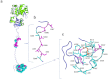
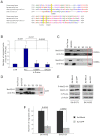
Nm23-H1 is one of the most interesting candidate genes for a relevant role in Neuroblastoma pathogenesis. H-Prune is the most characterized Nm23-H1 binding partner, and its overexpression has been shown in different human cancers. Our study focuses on the role of the Nm23-H1/h-Prune protein complex in Neuroblastoma. Using NMR spectroscopy, we performed a conformational analysis of the h-Prune C-terminal to identify the amino acids involved in the interaction with Nm23-H1. We developed a competitive permeable peptide (CPP) to impair the formation of the Nm23-H1/h-Prune complex and demonstrated that CPP causes impairment of cell motility, substantial impairment of tumor growth and metastases formation. Meta-analysis performed on three Neuroblastoma cohorts showed Nm23-H1 as the gene highly associated to Neuroblastoma aggressiveness. We also identified two other proteins (PTPRA and TRIM22) with expression levels significantly affected by CPP. These data suggest a new avenue for potential clinical application of CPP in Neuroblastoma treatment.
Figures


Similar articles
-
A therapeutic approach to treat prostate cancer by targeting Nm23-H1/h-Prune interaction.Naunyn Schmiedebergs Arch Pharmacol. 2015 Feb;388(2):257-69. doi: 10.1007/s00210-014-1035-8. Epub 2014 Aug 20. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25138575
-
The Nm23-H1-h-Prune complex in cellular physiology: a 'tip of the iceberg' protein network perspective.Mol Cell Biochem. 2009 Sep;329(1-2):149-59. doi: 10.1007/s11010-009-0115-4. Epub 2009 Apr 24. Mol Cell Biochem. 2009. PMID: 19390954 Review.
-
Phosphorylation of nm23-H1 by CKI induces its complex formation with h-prune and promotes cell motility.Oncogene. 2008 Mar 20;27(13):1853-64. doi: 10.1038/sj.onc.1210822. Epub 2007 Oct 1. Oncogene. 2008. PMID: 17906697
-
Amplification and overexpression of PRUNE in human sarcomas and breast carcinomas-a possible mechanism for altering the nm23-H1 activity.Oncogene. 2001 Oct 18;20(47):6881-90. doi: 10.1038/sj.onc.1204874. Oncogene. 2001. PMID: 11687967
-
Regulators affecting the metastasis suppressor activity of Nm23-H1.Mol Cell Biochem. 2009 Sep;329(1-2):167-73. doi: 10.1007/s11010-009-0109-2. Epub 2009 Apr 18. Mol Cell Biochem. 2009. PMID: 19377884 Review.
Cited by
-
General and specific promotion of flagellar assembly by a flagellar nucleoside diphosphate kinase.Mol Biol Cell. 2017 Nov 1;28(22):3029-3042. doi: 10.1091/mbc.E17-03-0156. Epub 2017 Sep 6. Mol Biol Cell. 2017. PMID: 28877983 Free PMC article.
-
The relationship of NM23 (NME) metastasis suppressor histidine phosphorylation to its nucleoside diphosphate kinase, histidine protein kinase and motility suppression activities.Oncotarget. 2017 Dec 31;9(12):10185-10202. doi: 10.18632/oncotarget.23796. eCollection 2018 Feb 13. Oncotarget. 2017. PMID: 29535799 Free PMC article.
-
Structure, Folding and Stability of Nucleoside Diphosphate Kinases.Int J Mol Sci. 2020 Sep 16;21(18):6779. doi: 10.3390/ijms21186779. Int J Mol Sci. 2020. PMID: 32947863 Free PMC article. Review.
-
The Potential Functional Roles of NME1 Histidine Kinase Activity in Neuroblastoma Pathogenesis.Int J Mol Sci. 2020 May 7;21(9):3319. doi: 10.3390/ijms21093319. Int J Mol Sci. 2020. PMID: 32392889 Free PMC article.
-
A therapeutic approach to treat prostate cancer by targeting Nm23-H1/h-Prune interaction.Naunyn Schmiedebergs Arch Pharmacol. 2015 Feb;388(2):257-69. doi: 10.1007/s00210-014-1035-8. Epub 2014 Aug 20. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25138575
References
-
- Brodeur G. M. et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 11, 1466–1477 (1993). - PubMed
-
- Breslow N. & McCann B. Statistical estimation of prognosis for children with neuroblastoma. Cancer research 31, 2098–2103 (1971). - PubMed
-
- Shimada H. et al. International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors: a report from the Children's Cancer Group. Cancer 92, 2451–2461 (2001). - PubMed
-
- Seeger R. C. et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. The New England journal of medicine 313, 1111–1116 (1985). - PubMed
-
- Look A. T. et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 9, 581–591 (1991). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

