Pentamines as substrate for human spermine oxidase
- PMID: 23449327
- PMCID: PMC3602902
- DOI: 10.1248/bpb.b12-00818
Pentamines as substrate for human spermine oxidase
Abstract
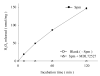
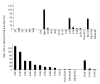
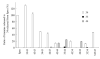
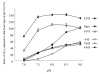
Substrate activities of various linear polyamines to human spermine oxidase (hSMO) were investigated. The activities were evaluated by monitoring the amount of H2O2 released from sample polyamines by hSMO. H2O2 was measured by a HPLC method that analyzed fluorescent dimers derived from the oxidation of homovanillic acid in the presence of horseradish peroxidase. Six triamines were tested and were found not to be hSMO substrates. Of sixteen tetramines tested, spermine (Spm) was the most active substrate, followed by homospermine and N-butylated Spm. Pentamines showed a characteristic pattern of substrate activity. Of thirteen pentamines tested, 3343 showed higher substrate activity than Spm, and 4343 showed similar activity to Spm. The activities of the other pentamines were as follows: 3443, 4443, 4344, 3344, 4334, 4444, and 3334 (in decreasing order). Product amines released from these pentamines by hSMO were then analyzed by HPLC. Triamine was the only observed product, and the amount of triamine was nearly equivalent to that of released H2O2. A marked difference in the pH dependency curves between tetramines and pentamines suggested that hSMO favored reactions with a non-protonated secondary nitrogen at the cleavage site. The Km and Vmax values for Spm and 3343 at pH 7.0 and 9.0 were consistent with the higher substrate activity of 3343 compared to Spm, as well as with the concept of a non-protonated secondary nitrogen at the cleavage site being preferred, and 3343 was well degraded at a physiological pH by hSMO.
Figures
Similar articles
-
Pentamine as a Substrate for Measuring Spermine Oxidase Activity.Methods Mol Biol. 2018;1694:149-154. doi: 10.1007/978-1-4939-7398-9_15. Methods Mol Biol. 2018. PMID: 29080165
-
Mechanistic studies of the yeast polyamine oxidase Fms1: kinetic mechanism, substrate specificity, and pH dependence.Biochemistry. 2010 Dec 14;49(49):10440-8. doi: 10.1021/bi1016099. Epub 2010 Nov 16. Biochemistry. 2010. PMID: 21067138 Free PMC article.
-
Purification and characterization of extracellular polyamine oxidase produced by Penicillium sp. no. PO-1.Biochim Biophys Acta. 1982 Jul 26;705(2):133-8. doi: 10.1016/0167-4838(82)90171-6. Biochim Biophys Acta. 1982. PMID: 7115736
-
Spermine metabolism and anticancer therapy.Curr Cancer Drug Targets. 2009 Mar;9(2):118-30. doi: 10.2174/156800909787580935. Curr Cancer Drug Targets. 2009. PMID: 19275753 Review.
-
Spermine oxidase: ten years after.Amino Acids. 2012 Feb;42(2-3):441-50. doi: 10.1007/s00726-011-1014-z. Epub 2011 Aug 2. Amino Acids. 2012. PMID: 21809080 Review.
Cited by
-
N1-Nonyl-1,4-diaminobutane ameliorates brain infarction size in photochemically induced thrombosis model mice.Neurosci Lett. 2018 Apr 13;672:118-122. doi: 10.1016/j.neulet.2018.01.054. Epub 2018 Feb 22. Neurosci Lett. 2018. PMID: 29477597 Free PMC article.
-
Identification of a Novel Substrate-Derived Spermine Oxidase Inhibitor.Acta Naturae. 2020 Jul-Sep;12(3):140-144. doi: 10.32607/actanaturae.10992. Acta Naturae. 2020. PMID: 33173604 Free PMC article.
-
Exploring the activity of polyamine analogues on polyamine and spermine oxidase: methoctramine, a potent and selective inhibitor of polyamine oxidase.J Enzyme Inhib Med Chem. 2019 Dec;34(1):740-752. doi: 10.1080/14756366.2019.1584620. J Enzyme Inhib Med Chem. 2019. PMID: 30829081 Free PMC article.
-
Structure-function relationships in the evolutionary framework of spermine oxidase.J Mol Evol. 2013 Jun;76(6):365-70. doi: 10.1007/s00239-013-9570-3. Epub 2013 Jul 5. J Mol Evol. 2013. PMID: 23828398 Review.
References
-
- Tabor CW. Tabor H. Polyamines. Annu. Rev. Biochem. 1984;53:749–790. - PubMed
-
- Wang Y, Devereux W, Woster PM, Murray-Stewart T, Hacker A, Casero RA. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001;61:5370–5373. - PubMed
-
- Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, Casero RA. Properties of purified recombinant human polyamine oxidase PAOh1/SMO. Biochem. Biophys. Res. Commun. 2003;304:605–611. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

