A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma
- PMID: 23449352
- PMCID: PMC3619271
- DOI: 10.1038/bjc.2013.85
A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma
Abstract
Background: Transarterial chemoembolisation (TACE) has not been shown to be superior to bland embolisation (TAE) for treatment of hepatocellular carcinoma (HCC).
Methods: We conducted a randomised phase II/III trial in patients with untreated HCC. Patients were randomised to TAE with polyvinyl alcohol (PVA) particles alone or sequential TACE (sTACE) in which cisplatin 50 mg was administered intrarterially 4-6 h before PVA embolisation. Treatment was repeated 3-weekly for up to three treatments. The primary endpoint was overall survival and secondary endpoints were progression-free survival, toxicity and response. Target sample sizes for phase II and III were 80 and 322.
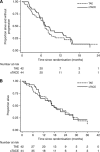
Results: The trial was terminated at phase II after 86 patients had been randomised. Patients were well matched for prognostic criteria. All three planned treatments were given to 57.1% (TAE) and 56.8% (TACE) patients. Grade 3/4 toxicity occurred in 63.5% and 83.7%, respectively (P=0.019). End-of-treatment RECIST response (CR+PR) was 13.2 and 32.6% (P=0.04) (mRECIST 47.3% and 67.4) and median overall survival and progression-free survival was 17.3 vs 16.3 (P=0.74) months and 7.2 vs 7.5 (P=0.59), respectively.
Conclusion: Transarterial chemoembolisation according this novel schedule is feasible and associated with a higher response rate than TAE alone. The survival benefit of TACE over TAE remains unproven.
Figures
References
-
- Asghar U, Meyer T. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer. J Hepatol. 2012;56 (3:686–695. - PubMed
-
- Brown KT, Gonen M, Gian Do K, Covey AM, Getrajdman GI, Zhao B, Sofocleous CT, Beattie C, DeMatteo RP, Solomon SB, Abou-Alfa GK.2012A randomized single blind controlled trial of beads versus doxorubicin-eluting beads for arterial embolization of hepatocellular carcinoma (HCC) J Clin Oncol 30(suppl 34abstract 143
-
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35 (3:421–430. - PubMed
-
- Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol. 2012;56 (6:1330–1335. - PubMed
-
- Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol. 2004;5 (7:409–418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous