Role of protein kinase C δ in ER stress and apoptosis induced by oxidized LDL in human vascular smooth muscle cells
- PMID: 23449456
- PMCID: PMC3734829
- DOI: 10.1038/cddis.2013.47
Role of protein kinase C δ in ER stress and apoptosis induced by oxidized LDL in human vascular smooth muscle cells
Abstract
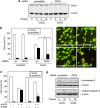
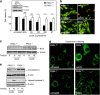
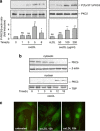
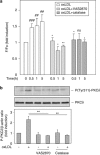
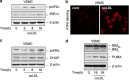
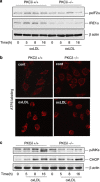
During atherogenesis, excess amounts of low-density lipoproteins (LDL) accumulate in the subendothelial space where they undergo oxidative modifications. Oxidized LDL (oxLDL) alter the fragile balance between survival and death of vascular smooth muscle cells (VSMC) thereby leading to plaque instability and finally to atherothrombotic events. As protein kinase C δ (PKCδ) is pro-apoptotic in many cell types, we investigated its potential role in the regulation of VSMC apoptosis induced by oxLDL. We found that human VSMC silenced for PKCδ exhibited a protection towards oxLDL-induced apoptosis. OxLDL triggered the activation of PKCδ as shown by its phosphorylation and nuclear translocation. PKCδ activation was dependent on the reactive oxygen species generated by oxLDL. Moreover, we demonstrated that PKCδ participates in oxLDL-induced endoplasmic reticulum (ER) stress-dependent apoptotic signaling mainly through the IRE1α/JNK pathway. Finally, the role of PKCδ in the development of atherosclerosis was supported by immunohistological analyses showing the colocalization of activated PKCδ with ER stress and lipid peroxidation markers in human atherosclerotic lesions. These findings highlight a role for PKCδ as a key regulator of oxLDL-induced ER stress-mediated apoptosis in VSMC, which may contribute to atherosclerotic plaque instability and rupture.
Figures


References
-
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. - PubMed
-
- Napoli C. Oxidation of LDL, atherogenesis, and apoptosis. Ann NY Acad Sci. 2003;1010:698–709. - PubMed
-
- Salvayre R, Auge N, Benoist H, Negre-Salvayre A. Oxidized low-density lipoprotein-induced apoptosis. Biochim Biophys Acta. 2002;1585:213–221. - PubMed
-
- Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. - PubMed
-
- Ingueneau C, Huynh-Do U, Thiers JC, Negre-Salvayre A, Salvayre R, Vindis C. Caveolin-1 sensitizes vascular smooth muscle cells to mildly oxidized LDL-induced apoptosis. Biochem Biophys Res Commun. 2008;369:889–893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

