The clinical impact of humoral immunity in pediatric renal transplantation
- PMID: 23449533
- PMCID: PMC3609135
- DOI: 10.1681/ASN.2012070663
The clinical impact of humoral immunity in pediatric renal transplantation
Abstract
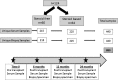
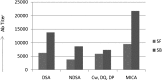
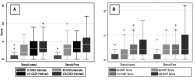
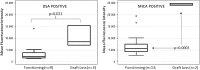
The development of anti-donor humoral responses after transplantation associates with higher risks for acute rejection and 1-year graft survival in adults, but the influence of humoral immunity on transplant outcomes in children is not well understood. Here, we studied the evolution of humoral immunity in low-risk pediatric patients during the first 2 years after renal transplantation. Using data from 130 pediatric renal transplant patients randomized to steroid-free (SF) or steroid-based (SB) immunosuppression in the NIH-SNSO1 trial, we correlated the presence of serum anti-HLA antibodies to donor HLA antigens (donor-specific antibodies) and serum MHC class 1-related chain A (MICA) antibody with both clinical outcomes and histology identified on protocol biopsies at 0, 6, 12, and 24 months. We detected de novo antibodies after transplant in 24% (23% of SF group and 25% of SB group), most often after the first year. Overall, 22% developed anti-HLA antibodies, of which 6% were donor-specific antibodies, and 6% developed anti-MICA antibody. Presence of these antibodies de novo associated with significantly higher risks for acute rejection (P=0.02), chronic graft injury (P=0.02), and decline in graft function (P=0.02). In summary, antibodies to HLA and MICA antigens appear in approximately 25% of unsensitized pediatric patients, placing them at greater risk for acute and chronic rejection with accelerated loss of graft function. Avoiding steroids does not seem to modify this incidence. Whether serial assessments of these antibodies after transplant could guide individual tailoring of immunosuppression requires additional study.
Figures


References
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B: Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4: 378–383, 2004 - PubMed
-
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000 - PubMed
-
- Zhang Q, Liang LW, Gjertson DW, Lassman C, Wilkinson AH, Kendrick E, Pham PT, Danovitch GM, Gritsch HA, Reed EF: Development of posttransplant antidonor HLA antibodies is associated with acute humoral rejection and early graft dysfunction. Transplantation 79: 591–598, 2005 - PubMed
-
- Terasaki PI, Ozawa M: Predicting kidney graft failure by HLA antibodies: A prospective trial. Am J Transplant 4: 438–443, 2004 - PubMed
-
- Loupy A, Hill GS, Jordan SC: The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348–357, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

