Noninvasive electrocardiographic imaging of arrhythmogenic substrates in humans
- PMID: 23449548
- PMCID: PMC3596167
- DOI: 10.1161/CIRCRESAHA.112.279315
Noninvasive electrocardiographic imaging of arrhythmogenic substrates in humans
Abstract
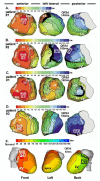
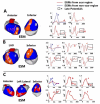
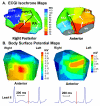
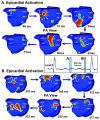
Cardiac excitation is determined by interactions between the source of electric activation (membrane depolarization) and the load that cardiac tissue presents. This relationship is altered in pathology by remodeling processes that often create a substrate favoring the development of cardiac arrhythmias. Most studies of arrhythmia mechanisms and arrhythmogenic substrates have been conducted in animal models, which may differ in important ways from the human pathologies they are designed to represent. Electrocardiographic imaging is a noninvasive method for mapping the electric activity of the heart in humans in real-world conditions. This review summarizes results from electrocardiographic imaging studies of arrhythmogenic substrates associated with human clinical arrhythmias. Examples include heart failure, myocardial infarction scar, atrial fibrillation, and abnormal ventricular repolarization.
Figures




References
-
- Rudy Y. Mathematical modeling of complex biological systems: from genes and molecules to organs and organisms: heart. Simulation and Modeling. In: Weinstein H, Egelman EH, editors. Comprehensive Biophysics. Vol. 9. Academic Press; Oxford: 2012. pp. 268–327.
-
- Ursell PC, Gardner PI, Albana A, Fenoglio JJ, Jr, Wit AL. Structural and electrophysiological changes in epicardial border zone of canine myocardial infarcts during healing. Circ Res. 1985;56:436–451. - PubMed
-
- Wit AL, Janse MJ. Experimental models of ventricular tachycardia and fibrillation caused by ischemia and infarction. Circulation. 1992;85:132–42. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

