Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy
- PMID: 23449602
- PMCID: PMC3571748
- DOI: 10.5665/sleep.2442
Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy
Abstract
Study objectives: Humans with narcolepsy and orexin/ataxin-3 transgenic (TG) mice exhibit extensive, but incomplete, degeneration of hypo-cretin (Hcrt) neurons. Partial Hcrt cell loss also occurs in Parkinson disease and other neurologic conditions. Whether Hcrt antagonists such as almorexant (ALM) can exert an effect on the Hcrt that remains after Hcrt neurodegeneration has not yet been determined. The current study was designed to evaluate the hypnotic and cataplexy-inducing efficacy of a Hcrt antagonist in an animal model with low Hcrt tone and compare the ALM efficacy profile in the disease model to that produced in wild-type (WT) control animals.
Design: Counterbalanced crossover study.
Setting: Home cage.
Patients or participants: Nine TG mice and 10 WT mice.
Interventions: ALM (30, 100, 300 mg/kg), vehicle and positive control injections, dark/active phase onset.
Measurements and results: During the 12-h dark period after dosing, ALM exacerbated cataplexy in TG mice and increased nonrapid eye movement sleep with heightened sleep/wake fragmentation in both genotypes. ALM showed greater hypnotic potency in WT mice than in TG mice. The 100 mg/kg dose conferred maximal promotion of cataplexy in TG mice and maximal promotion of REM sleep in WT mice. In TG mice, ALM (30 mg/ kg) paradoxically induced a transient increase in active wakefulness. Core body temperature (Tb) decreased after acute Hcrt receptor blockade, but the reduction in Tb that normally accompanies the wake-to-sleep transition was blunted in TG mice.
Conclusions: These complex dose- and genotype-dependent interactions underscore the importance of effector mechanisms downstream from Hcrt receptors that regulate arousal state. Cataplexy promotion by ALM warrants cautious use of Hcrt antagonists in patient populations with Hcrt neurodegeneration, but may also facilitate the discovery of anticataplectic medications.
Citation: Black SW; Morairty SR; Fisher SP; Chen TM; Warrier DR; Kilduff TS. Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy. SLEEP 2013;36(3):325-336.
Keywords: Cataplexy; hypocretin; mouse; narcolepsy; orexin.
Figures



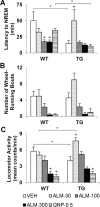



References
-
- Sakurai T, Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci. 2011;32:451–62. - PubMed
-
- Sinton CM. Orexin/hypocretin plays a role in the response to physiological disequilibrium. Sleep Med Rev. 2011;15:197–207. - PubMed
-
- Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. - PubMed
-
- Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. - PubMed
-
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

