MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis
- PMID: 23449618
- PMCID: PMC3804303
- DOI: 10.1530/JOE-12-0586
MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis
Abstract
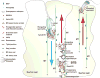
During spermatogenesis, spermatids derived from meiosis simultaneously undergo extensive morphological transformation, to become highly specialized and metabolically quiescent cells, and transport across the seminiferous epithelium. Spermatids are also transported back-and-forth across the seminiferous epithelium during the epithelial cycle until they line up at the luminal edge of the tubule to prepare for spermiation at stage VIII of the cycle. Spermatid transport thus requires the intricate coordination of the cytoskeletons in Sertoli cells (SCs) as spermatids are nonmotile cells lacking the ultrastructures of lamellipodia and filopodia, as well as the organized components of the cytoskeletons. In the course of preparing this brief review, we were surprised to see that, except for some earlier eminent morphological studies, little is known about the regulation of the microtubule (MT) cytoskeleton and the coordination of MT with the actin-based cytoskeleton to regulate spermatid transport during the epithelia cycle, illustrating that this is a largely neglected area of research in the field. Herein, we summarize recent findings in the field regarding the significance of actin- and tubulin-based cytoskeletons in SCs that support spermatid transport; we also highlight specific areas of research that deserve attention in future studies.
Conflict of interest statement
Figures
References
-
- Akhmanova A, Stehbens SJ, Yap AS. Touch, grasp, deliver and control: functional cross-talk between microtubules and cell adhesions. Traffic. 2009;10:268–274. - PubMed
-
- Amlani S, Vogl AW. Changes in the distribution of microtubules and intermediate filaments in mammalian Sertoli cells during spermatogenesis. Anat Rec. 1988;220:143–160. - PubMed
-
- Belletti B, Baldassarre G. Stathmin: a protein with many tasks. New biomarker and potential target in cancer. Expert Opin Ther Targets. 2011;15:1249–1266. - PubMed
-
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

